Research Article | DOI: https://doi.org/10.31579/ 2834-8788/006
Association between MTHFR and GSTP1 Polymorphisms and Ventricular Septal Defect in Iranian Subjects
- Mohammad Radgoudarzi 1
- Haleh Akhavan Niaki 2
- Ali Asghar Ahmadi 3
- Sepide Shariatnezhad 4
- Asma Javid 5*
1 Hazrat Rasoul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran.
2 Associate Professor, Department of Anatomy, Faculty of Medicine, Babol University of Medical Sciences, Babol, Iran.
3 North Research Center, Pasteur Institute of Iran, Amol, Iran.
4 Pediatric residents, Golestan Pediatric and neonatal research center, Golestan University of Medical Sci-ences, Gorgan, Iran.
5 Department of Pediatric, Firouzabadi Clinical Research Development Unit, Iran University of Medical Sci-ences, Tehran, Iran.
*Corresponding Author: Asma Javid, Department of Pediatric, Firouzabadi Clinical Research Development Unit, Iran University of Medical Sci-ences, Tehran, Iran
Citation: Radgoudarzi M., Haleh A. Niak, Ali A. Ahmadi, Shariatnezhad S. and Javid A. (2022). Assosiation between MTHFR and GSTP1 Polymorphisms and Ventricular Septal Defect in Iranian Subjects. Journal of Heart and Vasculature.1(2); DOI:10.31579/ 2834-8788/006
Copyright: © 2022 Asma Javid, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 03 November 2022 | Accepted: 11 November 2022 | Published: 24 November 2022
Keywords: MTHFR and GSTP1 Polymorphisms; Ventricular Septal Defect (VSD); CC, CT, and TT; Congenital heart defects (CHD)
Abstract
Ventricular septal defect (VSD) known as one of the most prevalent types of congenital heart defects (CHD). Both genetic and environmental risk factors are essential in its development. Methylenetetrahydrofolate reductase (MTHFR) is one of the fundamental regulatory enzymes in the homocysteine metabolic pathway, and related coding genes may play a crucial role in CHDs. GSTP1 genotypes codes Glutathione S-transferases (GSTs) play an essential role in detoxification and may increase the risk of DNA damage elicited by pesticide exposure.
This study determined the association of C677T and G1793A polymorphisms of the MTHFR gene and polymorphism of the GSTP1 gene in Iranian VSD subjects. A total of 98 children with VSDs and 89 healthy children were entered in this study. Genomic DNA was extracted from the blood samples of all the cases. The restriction fragment length polymorphism polymerase chain reaction (PCR-RFLP) method amplifies the C677T and G1793A and GSTP1 polymorphism.
The genotype frequencies of CC, CT, and TT of MTFHR C677T gene among the studied cases were 59 Percentage, 33 Percentage, and 7 Percentage, respectively, compared to 58.5 Percentage, 38 Percentage, and 3.5 Percentage controls.
The genotype frequencies of GG, GA, and AA of MTFHR G1793A gene among the studied cases were 97 Percentage, 0 Percentage, and 0 Percentage, respectively, compared to 93 Percentage, 7 Percentage, and 0 Percentage controls.
For the GSTP1 gene polymorphism, the frequencies of the genotypes of AA, AG, and GG among the cases were 42 Percentage, 51 Percentage, and 3 Percentage, respectively, while the frequencies were 40 Percentage, 49 Percentage, and 11 Percentage, respectively, among control subjects.
Significant differences were noticed (p less than 0.05) in AA VS. AG genotype between cases and control subjects.
1. Introduction
Congenital heart defects (CHD) are reported as the most frequent type of congenital anomalies. Universal recorded CHD prevalence increased in recent years from 0.6 before 1935 to 0.9 in 1000 live delivery, and the most significant belongs to Asia, with 9.3 per 1,000 live births [1]. Currency of CHD and severe forms of it has risen by 11 Percentage and 19 Percentage after 2000, respectively [2].
The mean prevalence of CHD has been estimated at 12.30 per 1000 live births, with a yearly prevalence of 17.51 per 1000 live births in IRAN [3].
Overall ventricular septal defects account for up to 40 Percentage of all congenital cardiac malformations, and it is the most frequent of CHDs (27 Percentage) in IRAN [4,5].
The etiology of VSD is primarily unexplained. Genetic and environmental factors may have a role. Most ventricular septal defects occur sporadically, with no apparent cause [6]. Some VSDs may have a genetic link causing heart problems to occur more frequently in certain families [7]. Familial compilation and twin research indicated susceptibility to this theory [8].
Completing the human genome project and the universal haplotype map project, converted single nucleotide polymorphisms (SNPs) to available markers to compute individuals' susceptibility to complicated illnesses, treatment efficiency, and adverse drug reactions [9-11]. This, in turn, facilitates clinicians and healthcare workers in taking proper actions. Difference methods have been affirmed for the genotyping of SNPs. Standard techniques include real-time PCR, DNA sequencing, restriction fragment length polymorphism (RFLP) analysis, and amplification refractory mutation system PCR (ARMS-PCR) [12-15].
Methylenetetrahydrofolate reductase (MTHFR) converts a molecule called 5, 10-methylenetetrahydrofolate (folic acid or vitamin B9) to 5-methyltetrahydrofolate (called levomefolic acid or active folate as well). Levomefolic acid can detoxify compounds in the body by moving its methyl group [16-17]. On the other hand, proper methylation enables the body to detoxify some potentially risky compounds generated by, or taken into, the body.
In the conversion of homocysteine to methionine, levomefolic acid or active folate is crucial. Methionine is necessary for producing glutathione, the body's main antioxidant product. Methionine is needed to produce myelin as well [18].
The body cannot produce homocysteine-derived products without this enzyme, and homocysteine upsurges in blood and other tissues. Homocysteine is mandatory for the production of cysteine, methionine, and other necessary mediators are needed for an assortment of metabolic processes like neurotransmitters dopamine, serotonin, and melatonin [19]. Raised homocysteine levels can lead to adverse affection on mental health and mood. Correlation of elevated homocysteine levels and birth defects, complex pregnancies, cardiovascular disease, high blood pressure, glaucoma, ischemic stroke, and atherosclerosis is reported [20, 21].
The MTHFR gene produces MTHFR and has been mapped to chromosome 1, region 1p36.3, and comprised 11 exons ranging in size from 102 to 432 bp [14, 15].
Several polymorphisms in the MTHFR gene have been identified. Because of researches, nearly half of the population may have an MTHFR gene mutation, and the two most problematic mutations are C677T and A1298C mutations, which denote the placement of the mutation on the gene [22].
Among them, A1298C (rs1801131) has been extensively considered, and its influence on DNA synthesis, genome stability, and sustaining proper homocysteine levels in the blood was demonstrated [23-26].
C677T is changing an alanine (A) into a valine (V) residue at codon 222 (A222 V) of the corresponding amino acid sequence, and several studies suggest this polymorphism performs an essential role in the etiology of stroke, neural tube defects, and congenital heart disease (CHD) [23 ,27-29].
Pishva and coworkers determined the association of C677T polymorphism of the MTHFR gene in Iranian VSD subjects. The frequencies for CC and CT genotypes of the MTHFR gene were 51.2 Percentage and 48.8 Percentage, respectively, in VSD patients compared to 56.8 Percentage and 43.2 Percentage, respectively, in control subjects [30]. But in Zhang T, study revealed no association between MTHFR C677T and A1298C polymorphisms and ventricular or atrial septal defect risk [31]. Kocakap BD results suggest that MTHFR 1298C allele is a risk factor for conotruncal heart disease [32].
In in-vitro experiments, Homozygosity for C677T, homozygosity for A1298C, and compound heterozygosity for A1298C and C677T are associated with a reduced enzyme activity of 45, 68, and 41 Percentage, respectively [33].
Recently, a novel polymorphic site, G1793A, in exon 11, prompting an arginine (R) to glutamine (Q) switch (R594Q) were identified [34]. Some studies repost its function on cardiovascular disease, and the theory of role-playing in CHD is concerned [35]. However, its universal distribution, especially in our country, was not adequately analyzed.
Another gene, GSTP1, codes Glutathione S-transferases (GSTs), a family of enzymes that function in xenobiotic metabolism and play an essential role in detoxification.
Studies propound the susceptibility of its relationship with childhood asthma, CAD in patients with type 2 diabetes mellitus, and malignancies as breast cancer and hepatocellular carcinoma [35- 37]. The soluble GSTs are classified into four main classes: alpha, mu, pi, and theta [38].
Fetal inherited GSTP1 Ile105Val polymorphism may modify the metabolism and excretion of xenobiotics from fetal tissue and raise the risk of congenital heart disease (CHD). Studies aimed to analyze the effects of GSTP1 genetic polymorphism (Ile105Val), and maternal environmental exposure on CHD risk revealed no significant differences in Ile105Val genotype frequencies between the children with CHD and the healthy children and no evidence of meaningful interaction between the maternal exposures and GSTP1 polymorphism [21].
No studies used a broad group of individuals to determine the frequency of the three genotypes within the general populations. In this study, we investigated the allelic frequencies of the C677T and G1793A polymorphism of the MTHFR gene and GSTP1 genotypes in 98 Iranian children.
2. Materials and Methods
Our study is a case-control study that considers patients younger than 11 years old with perimembranous or muscular VSD diagnosed at the Pediatric Cardiology Unit of Taleghani children's Hospital in Gorgan, Iran, during April 2016- 2018. The appropriate local authority ethically approves it.
2.1. Subject Recruitment
A total of 187 subjects were categorized into two comparative groups, 98 VSD patients versus 89 control cases. Control subjects were selected from 89 age- and sex-matched children admitted to hospital for elective surgery, and outpatient non-cardiac patients from the same geographic area following clinical assessment included thorough history taking and complete physical evaluation.
Family history, nationality, mother's age, mother's history, birth weight, familial marriage, and extra-cardiac anomalies were evaluated in all cases. In both groups, patients, and controls, information was obtained by a questionnaire. The demographic data were showed in Table 1 in detail.
| Characteristic | Patient | Control | P-value |
| Age (month) | 17.07 ±24.59 | 18.13 ±19.67 | P=0.124 |
| Mother's age (year) | 28.26 ±5.7 | 29.46 ±5.22 | P= 0.127 |
| Birth Weight(Kg) | 3.05 ±0.81 | 3.18 ±0.56 | P= 0.177 |
| Weight(Kg) | 8.43 ±5.73 | 9.57 ±3.98 | P=0.129 |
| Gender, male/female | 44/54 | 41/48 | P= 0.887 |
| Familial marriage, yes/no | 29/69 | 27/62 | P= 1 |
| Familial history, yes/no | 12/86 | 0/89 | P≤0.001 |
| Extra Cardiac, yes/no | 2/96 | 1/88 | P= 0.373 |
| Mother's history, yes/no | 8/90 | 1/88 | P≤0.05 |
Table 1.Demographic characteristics of study subjects
Cardiac evaluation of both groups included 12 lead electrocardiograms (ECG), chest radiography, and transthoracic echocardiography. In all cases, diagnoses were confirmed by Color-Doppler echocardiography.
All specimens were collected with informed consent from the participants' executor. DNA samples were prepared from peripheral blood samples anti-coagulated with ACD, using the standard phenol-chloroform extraction method.
Exclusion criteria were significant CHDs (such as single ventricle, double outlet RV, truncus arteriosus), distinct syndromic associations as VACTERLS, and recognized chromosomal anomaly including trisomy 18 or 21.
2.2. Genotyping assay
To determine the genotypes of MTHFR and GSTP1 genes, genomic DNA was magnified first by the respective primers using the polymerase chain reaction (PCR) technique. The PCR amplification for all the individual polymorphisms was done in a total volume of a 25 μL reaction mix consisting of 10 pmol of each primer and the Mastermix (i-DNA Biotechnology (M) Sdn Bhd, Kuchai Lama, Kuala Lumpur, Malaysia) and the template DNA. A negative control containing no genomic DNA and positive control of recognized genotype were always held in the set of reactions. All of the PCR cyclings were given to an iCycler machine (BioRad Laboratories, Hercules, CA, USA). The amplified PCR products for all the three gene polymorphisms were separated at 2 Percentage–4 Percentage agarose gel (Bioline, London, UK). The agarose gel was stained in ethidium bromide and visualized using Alpha Imager (Alpha Innotech, San Leandro, CA, USA). The PCR products of the respective genes were digested with 2–4 units of the individual restriction enzymes (Thermo Fisher Scientific, Inc, provided by Research Instruments Sdn Bhd, Petaling Jaya, Malaysia) with 10× Fast Digest Green Buffer in a final volume of 30 μL reaction mixture. Similar results were received when genotyping was performed for 15 Percentage of the specimens on two separate occasions.
2.3. Statistical Analysis
Data analysis was done via SPSS version 18.00 (SPSS Inc, South Wacker Drive, Chicago, IL, USA). Genotype and Alleles distribution were tested for deviation from the Hardy-Weinberg by a Chi-Square test. To illustrate the association, the odds ratio (OR) and its 95 Percentage confidence intervals (CI) were used and p less than 0.05 considered in all tests to be statistically significant.
3. Results
A total of 187 abstracts that met the inclusion criteria were retrieved through history taking and physical examination, 98 VSD children, and 89 normal controls.
The mean age was 17.07 ±24.59 in the study group and 18.13 ±19.67 in the control group. The mean weight of case subjects was 8.43 ±5.73, whereas the mean weight of controls was 11.57 ±3.98. The demographic data were listed in Table 1 in detail.
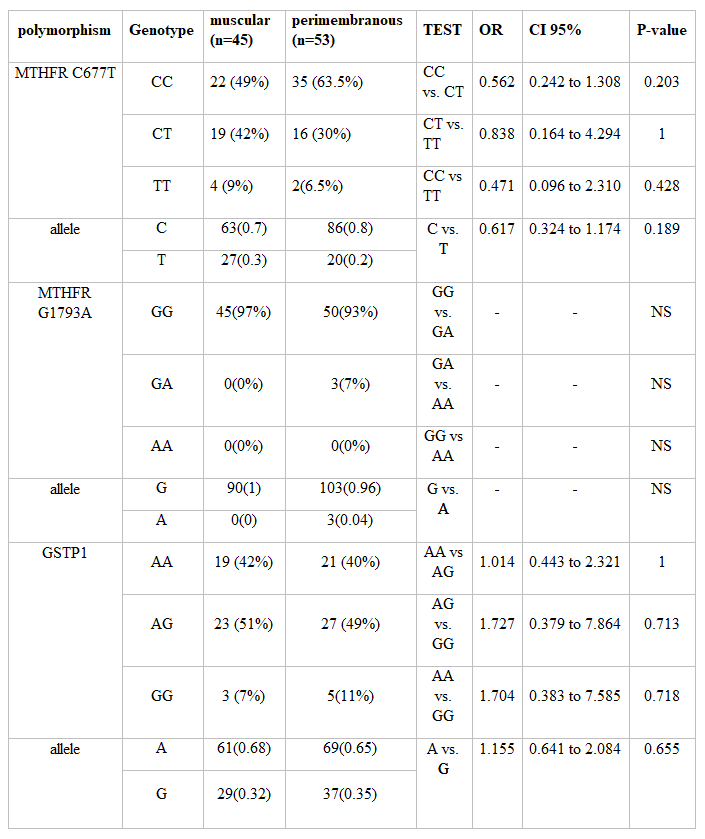
In childhood patients with VSD, the genotype frequencies of the MTHFR C677T polymorphism and C & T allele were as follows: TT 7, CT 33, CC 58, C 149, and T 47. Details of the two VSD groups, muscular and peri-membranous, were represented in Table 1.
In the control group, the homozygous MTHFR 677TT genotype was present in 3 (3.5 Percentage), the CT genotype in 34 (38 Percentage), and the CC genotype in 52 (58 Percentage) individuals. The resulting OR for patients carrying the homozygous TT genotype compared to the controls was 0.47 (CI, 0.11-1.9; P=0.34). Comparing VSD patients to the controls, the frequencies of MTHFR C677T genotypes did not show statistically significant differences between study groups (VSD subtypes and control).
The merge MTHFR C677T allele frequency determined using the random-effects model was 24 Percentage in the VSD patients and was 22 Percentage in the control. These were 76 Percentage in the VSD patients and were 78 Percentage in the control respectively for MTHFR –677C allele.
Among the 187 individuals, the MTHFR G1793A genotypes GG were 94 VSD & 82 control, GA was 4 VSD & 5 control, and AA was just two in the control group. The frequency of A allele of the MTHFR G1793A varied from .02 Percentage in the VSD group to .05 Percentage in control, and all of the populations in this study were in the Hardy–Weinberg law of equilibrium (P greater than 0.05). Details of the two VSD groups, muscular and peri-membranous, were represented in Table 3.
The resulting OR for patients carrying the homozygous AA genotype compared to the controls was not significant. The frequencies of MTHFR C1793A genotypes did not show statistically significant differences between different study groups (VSD subtypes and control). Interestingly, it was noted that 94 Percentage of patients had the GG genotype, but there was no significant difference between the two groups.
The pooled MTHFR G1793A allele frequency was 2 Percentage in the VSD patients and 5 Percentage in the control group. The frequencies for MTHFR –1793G allele were 98 Percentage and 95 Percentage in the VSD patients in the control group, respectively.
Frequencies of the GSTP1 polymorphism in VSD patients were as following AA 39, AG 50, GG 9, A 128 and G 68. In muscular VSD patient frequencies were as follows: AA, n=19 (42 Percentage); AG, n=23 (51 Percentage); GG, n=3 (7 Percentage); A, n=61(68 Percentage) and G, n=29(32 Percentage).In peri-membranous VSD patients were: AA, n=21 (40 Percentage); AG, n=27 (49 Percentage); GG, n=5 (11 Percentage); A, n=69(65 Percentage) and G, n=37(35 Percentage). Results of subgroup analyses are shown in the Tables 2 and Table 3.
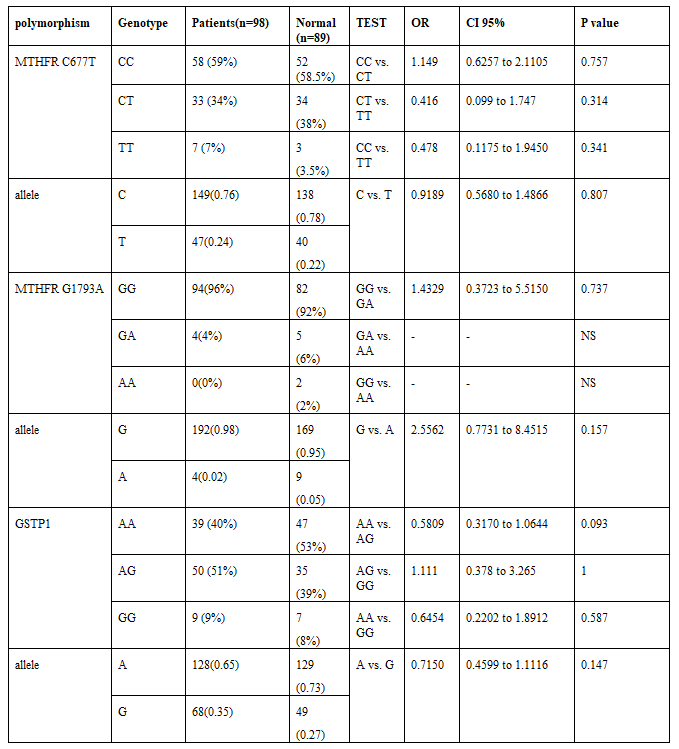
Moreover, there were no significant differences in the prevalence of alleles and genotypes between VSD patients with and without a family history of congenital heart defects.
4. Discussion
Congenital heart disease (CHD) is one of the most commonly noninfectious diseases, which compounds one-third of all congenital anomalies and is the main cause of birth defects lead in to infant mortality [39, 40]. The incidence of CHD in different studies varies from about 4/1,000 to 50/1,000 live births. The relative frequency of various significant forms of CHD also differs considerably from study to study. The prevalence of congenital cardiovascular malformations increased from 0.6 to 9.3 per 1,000 live births, and the prevalence of ventricular septal defect extended from 1.0 to 1.6 per 1,000 live births in the latest studies [1].
Ventricular septal defects are usually asymptomatic and close spontaneously [41]. There are definite multifactorial causes for CHDs, especially VSD, in which both environmental and genetic risk factors are consequential in the development of CHD [42, 43]
Mutations in the encoding gene of the homeobox transcription factor NKX2-5 were mapped to chromosome 5q35were found to cause nonsyndromic congenital heart disease and atrioventricular conduction abnormalities [44]. The significance of genetic factors in the development of CHD is also supported by data from genome-wide association studies (GWASs).it have affirmed that a zone on chromosome 4p16 adjacent to the MSX1 and STX18 genes was correlated with the risk of atrial septal defect of ostium Secundum type (ASD2) [45]. Also revealed that rs2228638 in NRP1 on 10p11 significantly increased the risk of Tetralogy of Fallot (TOF) [46]. Abdul-Sater Z and et al. have formely shown that a tandem repeat in the intrinsic region of NFATC1 is associated with ventricular septal defects. after that, they showed for the first time a potential link between a mutation in NFATC1 and tricuspid valve atresia [47]. Xuan C et al. identified HOMEZ and PLAGL1 as pathogenic genes in Chinese patients with isolated ventricular septal defects (VSDs) [48, 49].
Maternal hyperhomocysteinemia association with an increased risk of CHDs first reported in 1999 [50]. Wenstrom first noted a correlation between MTHFR gene polymorphism and susceptibility to CHD [51]. other studies supported the MTHFR -677T allele as a susceptibility cause for CHD in the Asian population and the -1298C allele role in the Caucasian pediatric population.8 Even MTHFR 677CT genotype posed as an implicating factor and as a maternal risk factor for septal defects in children with Down syndrome [52]. A study done by Zhu et al. showed that the MTHFR C677T locus variation is associated with the occurrence of the atrial septal defect (ASD) and patent ductus arteriosus (PDA) [53].
The 5, 10-methylenetetrahydrofolate reductase (MTHFR) gene is placed on chromosome 1 at 1p36.3. The primary product of the MTHFR gene is a catalytically active 77 kDa protein that catalyzes the transformation of 5, 10-methylenetetrahydrofolate into 5-methyltetrahydrofolate, the primary circulating form of folate [8] A frequent C677T mutation (rs1801133) in the MTHFR gene has been described, which was associated with a 50 Percentage reduction of MTHFR enzyme activity, a rise in plasma homocysteine concentration, and a decrease in plasma folic acid concentration [54]. Homozygosity (TT) for C677T polymorphism is associated with higher homocysteine levels and lower serum folate concentration than heterozygosity (CT) or wild-type genotype (CC) [55, 56]. otherwise, it was mentioned that A1298C heterozygosity and homozygosity were associated neither with higher total nor lower folate plasma concentration [57, 58].
Although many studies propound the association between MTHFR (methylenetetrahydrofolate reductase) polymorphisms and VSD/ASD risk, the results are controversial. In one study, the association of heart defects with four polymorphisms in folate-related genes was examined. The AG and 66GG genotypes were associated with decline odds ratios for heart defects. Maternal MTHFR 1298AC genotype was associated with an increased odds ratio for aortic valve stenosis. No association between SLC19A1 c.80A greater than G or MTHFR c.677C greater than T and heart defect was found [59]. Wang and coworkers carried out a meta-analysis. They reported that the infant and maternal MTHFR C667T polymorphism association might be associated with an increased occurrence of CHD.46 By contrast, Mamasoula and coworkers indicated that the MTHFR C677T polymorphism is not linked with the risk of CHD [60]. Several studies with conflicting outcomes have been published to confirm an association between MTHFR and CHD. Later studies, however, do not support the theory of MTHFR acting as a risk factor for the development of CHD [15, 61, 62]. A meta-analysis suggested that MTHFR C677T and A1298C polymorphisms are not associated with ventricular or atrial septal defect risk [16]. while the others had previously stated that they had found (for the first time!) that the embryonal MTHFR 677TT genotype was significantly associated with developing structural congenital heart malformations during early pregnancy [63].
Glutathione-S-transferases or GSTs catalyze the conjugation of many hydrophobic and electrophilic compounds and play an essential role in detoxification. The dissolved GSTs are classified into four main: alpha, mu, pi, and theta.
It is proposed that the GSTM1 (del) and GSTP1 (Ile105Val) gene polymorphisms themselves are not associated with the risk of congenital malformations (CMs) in a newborn. However, smoking may increase the risk magnitude of the GSTP1 (Ile105Val) genotypes in the formation of CMs in a child [18]. Results suggest that individuals with susceptible metabolic GSTP1 genotypes may experience an increased risk of DNA damage elicited by pesticide exposure [64]. to clear up the genetic factors causing clinical differences in children with Down syndrome and assess possible maternal risk factors; the scientists have investigated GSTM1, GSTT1, GSTP1 gene polymorphisms. Still, the data indicated no relationship between detected GST polymorphisms with the risk of having an infant with Down syndrome [20].
One study revealed an increased incidence of the GSTP1 variant genotypes among myelodysplastic syndromes (MDS) patients, providing evidence for a potential pathogenic role of the GSTP1 polymorphism on de novo MDS risk [21].
Our study aimed to assess the correlation between polymorphisms in the methylenetetrahydrofolate reductase (MTHFR), Glutathione S-Transferase Pi 1(GSTP1) genes, and the risk for VSD in Iranian subjects through a case-control study. Thus, our primary purpose was to figure out whether the MTHFR and GSTP1 gene polymorphisms are risk factors or not for the development of VSD in Iranian subjects.
5. Conclusion
This study shows no association between the MTHFR gene and VSD subjects. However, the GSTP1 gene AA polymorphisms can be considered as protective factors for VSD in Iranian subjects. More extensive cohort studies on mothers and children with distinct sub-classes are required to address risk adequately.
Declarations
Ethics approval and consent to participate: the Ethics Committee of the Gorgan University of Medical Sciences approved the study.
Consent for publication: Not applicable.
Availability of data and materials: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests: The authors declare that they have no competing interests.
Funding:
There is no funding
References
- Van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA et al. (2011).Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. Journal of the American College of Cardiology.;58(21):2241-2247.
View at Publisher | View at Google Scholar - Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N et al. (2014). Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation.: CIRCULATIONAHA. 113.008396.
View at Publisher | View at Google Scholar - Joundishapour A. (2008). Prevalence of congenital heart disease in Iran: a clinical study. J Med Sci.;8(6):547-552.
View at Publisher | View at Google Scholar - Amel-Shahbaz S, Behjati-Ardakani M, Namayandeh SM, Vafaeenasab M, Andishmand A et al. (2014). The epidemiological aspects of congenital heart disease in central and southern district of Iran. Advanced biomedical research.;3.
View at Publisher | View at Google Scholar - Penny DJ, Vick III GW. (2011). Ventricular septal defect. The Lancet377(9771):1103-1112.
View at Publisher | View at Google Scholar - van de Laar I, Wessels M. (2017). Inheritance of Congenital Heart Disease. Pregnancy and Congenital Heart Disease: Springer; p. 51-65.
View at Publisher | View at Google Scholar - Lage K, Greenway SC, Rosenfeld JA, Wakimoto H, Gorham JM et al. (2012). Genetic and environmental risk factors in congenital heart disease functionally converge in protein networks driving heart development. Proceedings of the National Academy of Sciences.201210730.
View at Publisher | View at Google Scholar - Xuan C, Li H, Zhao J-X, Wang H-W, Wang Y et al. (2014). Association between MTHFR polymorphisms and congenital heart disease: a meta-analysis based on 9,329 cases and 15,076 controls. Scientific reports.4:7311.
View at Publisher | View at Google Scholar - Erichsen HC, Chanock SJ. (2004). SNPs in cancer research and treatment. Br. J. Cancer 90, 747–751.
View at Publisher | View at Google Scholar - Sjöqvist F. (1999). The past, present, and future of clinical pharmacology. Eur. J. Clin. Pharmacol.8,553–557.
View at Publisher | View at Google Scholar - Wiechec E, Hansen LL. (2009). The effect of genetic variability on drug response in conventional breast cancer treatment. Eur. J. Pharmacol. 625, 122–130.
View at Publisher | View at Google Scholar - Ulvik A, Ueland PM. (2001). Single nucleotide polymorphism (SNP) genotyping in unprocessed whole blood and serum by real-time PCR, application to SNPs affecting homocysteine and folate metabolism. Clin. Chem. 47, 2050–2053.
View at Publisher | View at Google Scholar - Ronaghi M. (2001). Pyrosequencing sheds light on DNA sequencing. Genome Res. 11, 3–11.
View at Publisher | View at Google Scholar - Fukuen S, Fukuda T, Maune H Et al. (2002). Novel detection assay by PCR-RFLP and frequency of the CYP3A5 SNPs, CYP3A5*3 and *6, in a Japanese population. Pharmacogenetics 12, 331–334.
View at Publisher | View at Google Scholar - Newton CR. (1989). Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 7, 2503–2516.
View at Publisher | View at Google Scholar - Karakuła H, Opolska A, Kowal A, Domański M, Płotka A et al. (2009). Does diet affect our mood? The significance of folic acid and homocysteine. Polski Merkuriusz Lekarski: Organ Polskiego Towarzystwa Lekarskiego.26(152):136-141.
View at Publisher | View at Google Scholar - Goyette P, Pai A, Milos R et al. (1998). Gene structure of human and mouse methylenetetrahydrofolate reductase (MTHFR). Mamm Genome 8, 652–656.
View at Publisher | View at Google Scholar - Cristalli CP, Zannini C, Comai G, Baraldi O, Cuna V et al. (2017). Methylenetetrahydrofolate reductase, MTHFR, polymorphisms, and predisposition to different multifactorial disorders. Genes & Genomics. 39(7):689-699.
View at Publisher | View at Google Scholar - Jakubowski H. (2006). Pathophysiological consequences of homocysteine excess. The Journal of nutrition. 136(6):1741S-1749S.
View at Publisher | View at Google Scholar - Li P, Qin C. (2014). Methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and susceptibility to ischemic stroke: a meta-analysis. Gene. 535(2):359-364.
View at Publisher | View at Google Scholar - Azimova JE, Sergeev AV, Korobeynikova LA, Kondratieva NS, Kokaeva ZG et al. (2013). Effects of MTHFR gene polymorphism on the clinical and electrophysiological characteristics of migraine. BMC neurology.;13(1):103.
View at Publisher | View at Google Scholar - Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. (1998). A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Molecular genetics and metabolism.64(3):169-172.
View at Publisher | View at Google Scholar - Chen J, Gammon MD, Chan W et al. (2005). One-carbon metabolism, MTHFR polymorphisms, and risk of breast cancer. Cancer Res. 65, 1606–1614.
View at Publisher | View at Google Scholar - Weisberg IS, Jacques PF, Selhub J, et al. (2001). The 1298A→C polymorphism in methylenetetrahydrofolate reductase (MTHFR), in vitro expression and association with homocysteine. Atherosclerosis 156, 409–415.
View at Publisher | View at Google Scholar - Ueland PM, Hustad S, Schneede J, Refsum H, Vollset SE. (2001). Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol. Sci. 22, 195–201.
View at Publisher | View at Google Scholar - Gilbody S, Lewis S, Lightfoot T. (2007). Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders, a HuGE review. Am. J. Epidemiol. 165, 1–13.
View at Publisher | View at Google Scholar - Balta G, Yuksek N, Ozyurek E Et al. (2003). Characterization of MTHFR, GSTM1, GSTT1, GSTP1, and CYP1A1 genotypes in childhood acute leukemia. Am. J. Hematol. 73, 154–160.
View at Publisher | View at Google Scholar - De Marco P, Calevo MG, Moroni A Et al. (2002). Study of MTHFR and MS polymorphisms as risk factors for NTD in the Italian population. J. Hum. Genet. 47, 319–324. CAS.
View at Publisher | View at Google Scholar - Sayin Kocakap BD, Sanli C, Cabuk F, Koc M, Kutsal A. (2015). Association of MTHFR A1298C polymorphism with conotruncal heart disease. Cardiol. Young. 25, 1326–1331.
View at Publisher | View at Google Scholar - Pishva SR, Vasudevan R, Etemad A, Heidari F, Komara M et al. (2013). Analysis of MTHFR and MTRR gene polymorphisms in Iranian ventricular septal defect subjects. International Journal of molecular sciences. 14(2):2739-2752.
View at Publisher | View at Google Scholar - Zhang T, Wu Q. (2016). MTHFR C677T and A1298C polymorphisms are not related to ventricular or atrial septal defect: a meta-analysis of 1272 cases and 1386 controls. INTERNATIONAL JOURNAL OF CLINICAL AND EXPERIMENTAL MEDICINE. 9(6):10673-10683.
View at Publisher | View at Google Scholar - Kocakap BDS, Sanli C, Cabuk F, Koc M, Kutsal A. (2015). Association of MTHFR A1298C polymorphism with conotruncal heart disease. Cardiology in the Young. 25(7):1326-1331.
View at Publisher | View at Google Scholar - Weisberg IS, Jacques PF, Selhub J, Bostom AG, Chen Z et al. (2001). The 1298A→C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression, association with homocysteine. Atherosclerosis. 156(2):409–415.
View at Publisher | View at Google Scholar - Rady PL, Szucs S, Grady J, Hudnall SD, Kellner LH et al. (2002). Genetic polymorphisms of methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) in ethnic populations in Texas; a report of a novel MTHFR polymorphic site, G1793A. Am J Med Genet. 107(2):162–168.
View at Publisher | View at Google Scholar - Xu WH, Shrubsole MJ, Xiang YB, Cai Q, Zhao GM et al. (2007). Dietary folate intake, MTHFR genetic polymorphisms, and the risk of endometrial cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 16(2):281–287.
View at Publisher | View at Google Scholar - Chen Y-L, Tseng H-S, Kuo W-H, Yang S-F, Chen D-R et al. (2010). Glutathione S-Transferase P1 (GSTP1) gene polymorphism increases age-related susceptibility to hepatocellular carcinoma. BMC medical genetics. 11(1):46.
View at Publisher | View at Google Scholar - Ramprasath T, Murugan PS, Prabakaran AD, Gomathi P, Rathinavel A et al. (2011). Potential risk modifications of GSTT1, GSTM1 and GSTP1 (Glutathione-S-transferases) variants and their association to CAD in patients with type-2 diabetes. Biochemical and biophysical research communications. 407(1):49-53.
View at Publisher | View at Google Scholar - Kamada F, Mashimo Y, Inoue H, Shao C, Hirota T et al. (2007). The GSTP1 gene is a susceptibility gene for childhood asthma and the GSTM1 gene is a modifier of the GSTP1 gene. International archives of allergy and immunology. 144(4):275-286.
View at Publisher | View at Google Scholar - Liu C-x, Shen A, Li X-f, Jiao W-w, Bai S et al. (2009). Association of TBX5 gene polymorphism with ventricular septal defect in the Chinese Han population. Chinese medical Journal. 122(1):30-34.
View at Publisher | View at Google Scholar - Van Beynum I, Den Heijer M, Blom H, Kapusta L. (2007). The MTHFR 677C→ T polymorphism and the risk of congenital heart defects: a literature review and meta-analysis. QJM: An International Journal of Medicine. 100(12):743-753.
View at Publisher | View at Google Scholar - Srivastava D, Olson EN. (2000). A genetic blueprint for cardiac development. Nature. 407(6801):221.
View at Publisher | View at Google Scholar - Bruneau BG. (2008). The developmental genetics of congenital heart disease. Nature. 451(7181):943.
View at Publisher | View at Google Scholar - Schott J-J, Benson DW, Basson CT, Pease W, Silberbach GM et al. (1998). Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 281(5373):108-111.
View at Publisher | View at Google Scholar - Cordell HJ, Bentham J, Topf A, Zelenika D, Heath S et al. (2013). Genome-wide association study of multiple congenital heart disease phenotypes identifies a susceptibility locus for atrial septal defect at chromosome 4p16. Nature genetics. 45(7):822.
View at Publisher | View at Google Scholar - Xu J, Lin Y, Si L, Jin G, Dai J et al. (2014). Genetic variants at 10p11 confer risk of Tetralogy of Fallot in Chinese of Nanjing. PloS one. 9(3):e89636.
View at Publisher | View at Google Scholar - Abdul-Sater Z, Yehya A, Beresian J, Salem E, Kamar A et al. (2012). Two heterozygous mutations in NFATC1 in a patient with Tricuspid Atresia. PloS one. 7(11):e49532.
View at Publisher | View at Google Scholar - Xuan C, Wang B-B, Gao G, Bai X-Y, Yang Q et al. (2012). A novel variation of PLAGL1 in Chinese patients with isolated ventricular septal defect. Genetic testing and molecular biomarkers. 16(8):984-987.
View at Publisher | View at Google Scholar - Xuan C, Jia K-G, Wang B-B, Bai X-Y, Gao G et al. (2013). Identification of two novel mutations of the HOMEZ gene in Chinese patients with isolated ventricular septal defect. Genetic testing and molecular biomarkers. 17(5):390-394.
View at Publisher | View at Google Scholar - Kapusta L, Haagmans ML, Steegers EA, Cuypers MH, Blom HJ et al. (1999). Congenital heart defects and maternal derangement of homocysteine metabolism. The Journal of pediatrics. 135(6):773-774.
View at Publisher | View at Google Scholar - Lee C-N, Su Y-N, Cheng W-F, Lin M-T, Wang J-K et al. (2005). Association of the C677T methylenetetrahydrofolate reductase mutation with congenital heart diseases. Acta obstetricia et Gynecologica Scandinavica. 84(12):1134-1140.
View at Publisher | View at Google Scholar - Elsayed GM, Elsayed SM, Ezz-Elarab SS. (2014). Maternal MTHFR C677T genotype and septal defects in offspring with Down syndrome: A pilot study. Egyptian Journal of Medical Human Genetics. 15(1):39-44.
View at Publisher | View at Google Scholar - Zhu WL, Li Y, Yan L, Dao J, Li S. (2005). Maternal and offspring MTHFR gene C677T polymorphism as predictors of congenital atrial septal defect and patent ductus arteriosus. Molecular human reproduction. 12(1):51-54.
View at Publisher | View at Google Scholar - Biselli PM, Guerzoni AR, de Godoy MF, Eberlin MN, Haddad R et al. (2010). Genetic polymorphisms involved in folate metabolism and concentrations of methylmalonic acid and folate on plasma homocysteine and risk of coronary artery disease. Journal of thrombosis and thrombolysis. 29(1):32.
View at Publisher | View at Google Scholar - van der Put NM, Trijbels F, van den Heuvel L, Blom H, Steegers-Theunissen R et al. (1995). Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. The Lancet. 346(8982):1070-1071.
View at Publisher | View at Google Scholar - Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH et al. (1996). Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation.93(1):7-9.
View at Publisher | View at Google Scholar - Xuan C, Lun L-M. (2013). Association between the methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to preeclampsia: the need for data clarification in a recent meta-analysis. Hypertension Research. 36(5):463.
View at Publisher | View at Google Scholar - Xuan C, Bai X-Y, Gao G, Yang Q, He G-W. Association between polymorphism of methylenetetrahydrofolate reductase (MTHFR) C677T and risk of myocardial infarction: a meta-analysis for 8,140 cases and 10,522 controls. Archives of medical research. 2011;42(8):677-685.
View at Publisher | View at Google Scholar - Christensen KE, Zada YF, Rohlicek CV, Andelfinger GU, Michaud JLet al. Risk of congenital heart defects is influenced by genetic variation in folate metabolism. Cardiology in the Young. 2013;23(1):89-98.
View at Publisher | View at Google Scholar - Wang W, Wang Y, Gong F, Zhu W, Fu S. (2013). MTHFR C677T polymorphism and risk of congenital heart defects: evidence from 29 case-control and TDT studies. Plos One. 2013;8(3):e58041.
View at Publisher | View at Google Scholar - Mamasoula C, Prentice RR, Pierscionek T, Pangilinan F, Mills JL et al. (2013). Association Between C677T Polymorphism of Methylene Tetrahydrofolate Reductase and Congenital Heart DiseaseClinical Perspective: Meta-Analysis of 7697 Cases and 13 125 Controls. Circulation: Genomic and Precision Medicine. 6(4):347-353.
View at Publisher | View at Google Scholar - Wenstrom KD, Johanning GL, Johnston KE, DuBard M. (2001). Association of the C677T methylenetetrahydrofolate reductase mutation and elevated homocysteine levels with congenital cardiac malformations. American Journal of obstetrics and gynecology. 184(5):806-817.
View at Publisher | View at Google Scholar - Shaw GM, Iovannisci DM, Yang W, Finnell RH, Carmichael SL et al. (2005). Risks of human conotruncal heart defects associated with 32 single nucleotide polymorphisms of selected cardiovascular disease‐related genes. American Journal of Medical Genetics Part A.138(1):21-26.
View at Publisher | View at Google Scholar - Junker R, Kotthoff S, Vielhaber H, Halimeh S, Kosch A et al. (2001). Infant methylenetetrahydrofolate reductase 677TT genotype is a risk factor for congenital heart disease. Cardiovascular research.51(2):251-254.
View at Publisher | View at Google Scholar - Frosst P, Blom H, Milos R, Goyette P, Sheppard CA et al. (1995). A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nature genetics.10(1):111.
View at Publisher | View at Google Scholar

 Clinic
Clinic
