Research Article | DOI: https://doi.org/10.31579/2834-8389/011
Again, About the Ability of Positively Charged Water to Promote Cell Division
- Yuri Pivovarenko *
Research and Training Center ‘Physical and Chemical Materials Science’ Under Kyiv Taras Shevchenko University and NAS of Ukraine, Kiev, Ukraine
*Corresponding Author: Yuri Pivovarenko. Research and Training Center ‘Physical and Chemical Materials Science’ Under Kyiv Taras Shevchenko University and NAS of Ukraine, Kiev, Ukraine
Citation: Yuri Pivovarenko, (2024), Again, About the Ability of Positively Charged Water to Promote Cell Division. International Journal of Clinical Case Reports, 3(1); DOI:10.31579/2834-8389/011
Copyright: © 2024 Yuri Pivovarenko. This is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 21 December 2023 | Accepted: 02 January 2024 | Published: 09 January 2024
Keywords: water; fats; interactions; cell division
Abstract
It was previously established that the electric potential of water is an indicator that reflects its properties, including those of interest to both physicians and biologists. So, in experiments with biopolymers, including DNA, and salts that form crystal hydrates, it was shown that water with a positive potential has high penetrating and hydrating capabilities. However, it was shown that water with a negative potential not only has a low penetrating capability, but also promotes the dehydration of both biopolymers and the indicated salts. The specified differences in the properties of oppositely charged waters allowed assuming that water with a positive potential promotes cell division, unlike water with a negative potential. This assumption was based on the fact that water is the main cellular component, and therefore its accumulation by cells must precede their division; in addition, this very assumption took into account the fact that it is positively charged water that is able to hydrate the polymeric and salt components of cells and, thus, be retained by them. Accordingly, it has been assumed that the dehydrating properties of negatively charged water cause its blocking effect on cell division. Over time, it was realized that the lack of data on the interactions of oppositely charged waters with fats reduces the reliability of the proposed assumptions. For this reason, interactions of oppositely charged waters with fats and oils have been further studied. Thus, it was shown (literally) that positively charged water actively interacts with oils of animal and vegetable origin, while negatively charged water does not interact with them. In particular, it was shown that stable emulsions are formed by mixing oils with positively charged water and are not formed by mixing them with negatively charged water. However, it was concluded that the obtained visualizations do not allow detecting possible chemical interactions of oils with positively charged water; since it is precisely such interactions that should be of particular interest to oncologists, they are discussed separately.
Introduction
It was previously established that water with a positive electrical potential better hydrates salt capable of forming crystal hydrates than water with a negative electrical potential [1, 2]. In the different hydrating abilities of oppositely charged waters with respect to such salts, it is easiest made sure by using copper sulfate (Figures 1, 2); this easiness is due to the fact that the intensity of the blue color of copper sulfate corresponds to the degree of its hydration [3]. Both the higher surface tension of positively charged water and the lower surface tension of negatively charged water were also confirmed in the mentioned experiments with salts (Figure 1) [1].

The intensity of the blue color of CuSO4reflects the degree of its hydration [3].

The rhombic crystal used was diluted from the corresponding salt solution prepared on positively charged water (Figure 1, left) [1].
Soon after, it was established that water with a positive potential hydrates various biopolymer no less efficiently than the mentioned salts, while water with a negative electrical potential rather dehydrates the same biopolymers [1, 2, 4]; the different hydrating abilities of oppositely charged waters in relation to biopolymers can be verified by the example of starch (Figure 3). It is worth noting right here that the abnormally high penetrating power of positively charged water was also found in experiments with biopolymers (Figure 3) [1, 2, 4].
Obviously, these (Figures 1 – 3) and similar results ultimately allowed assuming that water with a positive potential promotes cell division, unlike water with a negative potential [2, 4]. This assumption was based on the fact that water is the main cellular component, and therefore its accumulation by cells must precede their division; in addition, this very assumption took into account the fact that it is positively charged water that is able to hydrate the polymeric and salt components of cells and, thus, be retained by them. Accordingly, it has been assumed that the dehydrating properties of negatively charged water cause its blocking effect on cell division. Subsequently, it became clear that the interactions of oppositely charged waters with fats should also be considered in terms of the proposed assumptions.

Water with negative potential was obtained by bubbling uncharged water with hydrogen gas (left); water with a positive potential was obtained by bubbling uncharged water with gaseous oxygen (right).
Water with a positive potential quickly evaporates even from a closed plastic flagon: the arrow shows the daily decreased in the level of this water.
Both water used had 20 – 22 °C [1, 2, 4].
With this in mind, these interactions have been studied to some extent. The results of these studies and their discussion are presented here for your attention; probably, all this will interest first of all oncologists.
Materials and methods
Water with the required electric charge (potential) was obtained as in [1].
To UV spectra recording Specord UV VIS (Carl Zeiss Jena, Germany) was used.
Cod liver oil from “De Luxe” (Iceland) was used. Refined sunflower oil from “Svoja Linija” (Ukraine) was also used.
Other reagents were purchased from “Ukrreachim” (Ukraine).
Results
First, it was established that positively charged water transforms oil drops into films (iridescent under suitable lighting) almost instantly (Figure 4).
However, it was established that negatively charged water does not change the shapes of oil drops applied to its surface (Figures 5, 6).

Thus, the given results (Figures 4 – 6) showed that positively charged water literally dilutes the oils, while negatively charged water does not interact with them.
In order to gain a deeper understanding of the nature of the interactions between oils and electrized water, an attempt was made to determine the distribution of electric charges in aqueous media that are in contact with oils. Given the sensitivity of the color of copper sulfate to the electrization of its aqueous environment (Figures 1, 2), aqueous solutions of this salt were used as an indicator. Thus, it was established that intensely blue compact crystals are concentrated in those areas of dried copper sulfate aqueous solutions that are in direct contact with oils, while pale blue plant crystals are formed in those areas of these very dried solutions that are distant from the oils (Figure 7); as has been shown (Figures 1, 2), this distribution of crystals with different intensity of blue color means positive electrization of water environments that are in direct contact with oils, and negative electrization of water environments that are distant from oils.

To better understand the reason for this distribution of electric charges, it is worth applying Kyon’s rule: upon contact of two phases, the phase with a higher dielectric permeability acquires a positive charge, while the phase with a lower dielectric permeability acquires a negative charge [3]. Since the dielectric permeability of oils is significantly less than the dielectric permeability of water [3, 5], oils in contact with water acquire a negative charge, while water in contact with oils acquires a positive charge.
Obviously, right now it is appropriate to realize that this very arrangement of crystals, which differ in intensity of blue color (Figure 7), actually visualizes the structure of a typical electric double layer (Figure 8) [6].
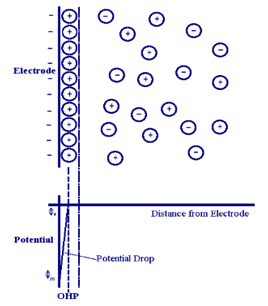
Taking into account all this (Figures 7 and 8) and the existing theory [6], it was assumed that the positively charged components of water (actually protons and oxonium ions, i.e., hydrated protons [3]) are of key importance for the formation of colloidal particles of oils, that is, for the emulsification of oils in water. This supposition was tested experimentally.
During such testing, it was established that suspensions formed by intensive mixing of oils with positively charged water do not stratify for hours (Figure 9, left), unlike suspensions formed by intensive mixing of the same oils with negatively charged water, which stratify within minutes (Figure 9, right).

Among other things, the obtained results force us to clarify such concepts as hydrophilicity and hydrophobicity, as well as the concept of surface activity [6]; in this aspect, the results of spectral studies look important (Figure 10).
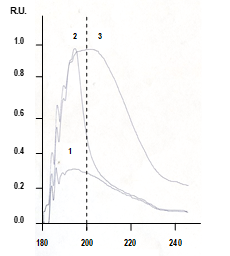
Thus, it was established that the UV absorption spectra of aqueous solutions of surface-inactive substances (such as KCl, NaCl, ammoniac, glycerin, inorganic acids, potassium and sodium hydroxides) look the same as the UV absorption spectra of negatively charged water (Figure 10, spectrum 2), while the UV absorption spectra of surface-active substances (such as methanol, ethanol, sodium lauryl sulfate, sodium palmitate, sodium stearate) look the same as the UV absorption spectra of positively charged water (Figure 10, spectrum 3).
Be that as it may, these very spectra (Figure 10) made it possible to more consciously compare the UV absorption spectra of lymphocyte suspensions of healthy people (Figure 11, spectra 1) and patients with chronic B-cell chronic lymphocytic leukemia (Figure 11, spectra 2) [7].
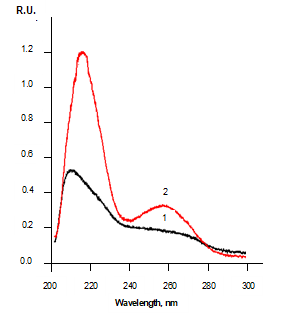
The UV absorption spectra of aqueous solutions of typical surface-inactive substances look the same as the UV absorption spectra of negatively charged water, while the UV absorption spectra of typical surface-active substances look the same as the UV absorption spectra of positively charged water. Moreover, these very spectra (Figure 10) made it possible to predict some affinity of negatively charged water with aqueous solutions of typical surface-inactive substances, as well as some affinity of positively charged water with aqueous solutions of typical surface-active substances; in the least case, an affinity is consistent with the ability of positively charged water to hydrate oils (Figure 9, left) in the same way as aqueous solutions of surfactants [6].
Discussion
Thus, it was shown (literally) that positively charged water actively interacts with oils of animal and vegetable origin and actually penetrates them, while negatively charged water does not interact with them. Moreover, the result presented in Figure 4 shows that the positively charged water actually dissolves the oils; this, in turn, shows that the similarity of the UV absorption spectra of positively charged water and aqueous solutions of surfactants (Figure 10, spectrum 3) is not accidental. However, the result presented in Figures 5 and 6 show that negatively charged water is indifferent to these very oils; this, in turn, shows that the similarity of the UV absorption spectra of negatively charged water and aqueous solutions of surface inactive substances (Figure 10, spectrum 2) is also not coincidental. Ultimately, all this is confirmed by the results presented in Figure 9.
Probably, it is also appropriate to pay attention to the visual, at least, similarity of the interactions of oppositely charged waters with oils (Figures 4 – 6) and powdered starch (Figure 12).

Be that as it may, all these results allows expecting that the positive electrization of the external
environment of cells will increase the fluidity of their membranes and, in this way, facilitate their division, while the negative electrization of the extracellular environment will at least not promote cell division. Thus, the assumption about the stimulating effect of positively charged water on cell division has received additional confirmations, as well as the assumption that negatively charged water at least slows down cell division [2]. Therefore, it can be considered that the initial goal of the conducted research has been achieved and be limited to this.
Unfortunately, the obtained results do not allowed detecting possible chemical interactions of oils with positively charged water. Since these very chemical interactions may be of interest to the readers of this journal, specifically to oncologists, they should be discussed separately. Thus, it is quite likely that the ability of positively charged water to hydrate oils (Figure 9, left) is due to the property of protons (in which this water is rich [1]) to catalyze the joining of water molecules to double bounds of oil molecules [5]:
–CH=CH– + H2O → –HCOH–CH2– (1).
Therefore, it is likely that positively charged water contributes to the appearance of hydrophilic hydrocarbon fragments in the composition of oil molecules. At least, existing ideas about the catalytic properties of hydrogen ions [8, 9] do not exclude such a possibility.
Accordingly, all this allows expecting that the external contact of cells with positively charged water will increase the hydrophilicity of their cytoplasmic membranes, at least. Consequently, it can also be expected that the positive electrization of the cellular environment will contribute to the swelling of cells, which is a prerequisite for their division. (It should also be expected that increasing the hydrophilicity of cytoplasmic membranes will increase their permeability to inorganic ions and glucose, which are necessary for both old and new cells [10, 11].
As well, it is known that the protons are directly involved in the formation of hydrogen peroxide from molecular oxygen:
О2 + 2е + 2Н+ → H2О2 (2) [12].
It is also known that alkenes are able to attach hydrogen peroxide, turning into diatomic alcohols:
–CH=CH– + H2О2 → –HCOH–HCOH– (3) [5, 8, 9].
Be that as it may, all these reactions (1) – (3) additionally indicate the possible participation of protons in the transformation of hydrophobic components of biological membranes, namely alkenes, into more hydrophilic, namely diatomic alcohols, with all the above consequences for cells.
Probably, the involvement of hydrogen peroxide in the formation of alkyl peroxides should be considered in the same aspect:
–CH2–CH=CH–CH2– + H2О2 → –HCOOH–CH=CH–CH2– (4) [8, 9].
Of course, the acidic properties of the latter, due to which they are able to dissociate with the formation of hydrophilic anions and the same protons [8, 9], should also not be overlooked, as well as the ability of protons to stimulate the decomposition of hydrogen peroxide to form atomic oxygen [3].
In view of this, the attempts of some oncologists to reduce the content of protons in the bodies of cancer patients do not seem unreasonable. Let’s consider some means used by oncologists for such attempts, that is, in accordance with the proposed concept.
Thus, this very concept suggests that it is the ability of dimethyl sulfoxide (DMSO) to bind protons determines both its ability to destructure water [3] and its antitumor effect. Accordingly, the same concept allows offering an alternative, at least an additional, explanation for the revealed antitumor effect of DMSO [13, 14].
As well, the possible epigenetic effects of negative electrization of the water environment of DNA [15], which undoubtedly occurs with the medical use of DMSO, should also be considered. However, the direct epigenetic effects of DMSO must also be due to its ability to replace its own H3C-radicals with OH-radicals [16, 17] that are part of 8-OH-Guanine (Figure 13) [18].
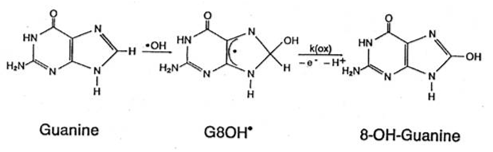
At the same time, it is bearing in mind the possible attachment of H3C-radicald that were part of DMSO to cytosines complementary to guanines, with the formation of 5-H3C-cytosines capable of blocking gene expression [19]; hence, it can be expected that this very methylation will prevent the division of cellular DNA and, consequently, cells. Nevertheless, the use of DMSO in antitumor therapy does not seem unreasonable.
As well, all of the above fully justifies the use of hydrogen gas by oncologists [20]. Thus, the chemical properties of hydrogen [3, 8, 9] suggest that it is able to restore not only oxidized nuclear DNA of cancer patients [21], but also their oxidized cytoplasmic membranes, thereby reducing the permeability of the latter to water. In particular, it can be expected that the permeability of the oxidized cytoplasmic membranes to water will decrease as a result of the following reaction:
R–HCOH–R + 2H* → R–CH2–R + H2O (5).
Probably, water saturated with prana [22], that is, electrons [23], should also be perceived as a means of hydrogen therapy, since electrons convert water protons into hydrogen atoms:
e + H+ → H* (6).
Conclusion
So, the assumption that water with a positive electric potential stimulates cell division, while water with a negative electric potential inhibits it [2, 4] has gained additional confirmations. In view of this, this assumption can no longer be perceived as completely groundless. This, in particular, gives reason to consider DMSO and hydrogen gas as means of neutralizing water protons (which contribute to cell division), that is, as anticancer agents. In addition, this allows considering negatively charged water as the most available antitumor agent, at least preventive; this availability is due to the ease of obtaining such water [1, 2, 22 – 24].
Be that as it may, water should not continue to be perceived as an inert medium; of course, this also applies to water, which is a component of the human body.
References
- Radesic B, Sharma A. (2004) Levonorgestrel-releasing intrauterine system for treating menstrual disorders: A patient satisfaction questionnaire. Aust NZ J Obstet Gynecol, 44: 247- 251.
View at Publisher | View at Google Scholar

 Clinic
Clinic
