Review Article | DOI: https://doi.org/10.31579/2834-8389/006
Vaccination against SARS-CoV-2: Efficacy and Side effects
1 Department of Microbiology, faculty of pharmacy, Alsham private university, Latakia, Syria, Department of biochemistry and microbiology, faculty of pharmacy, Tishreen university, Latakia, Syria
2 Department of Pharmaceutics, faculty of pharmacy, Alsham private university, Latakia, Syria.
3 Department of pathology, Faculty of medicine, Charles Drew University of medicine and science/ University of California Los Angeles (UCLA), USA.
*Corresponding Author: Rim M. Harfouch. Department of Microbiology, faculty of pharmacy, Alsham private university, Latakia, Syria, Department of biochemistry and microbiology, faculty of pharmacy, Tishreen university, Latakia, Syria.
Citation: Rim M. Harfouch, Lama Al Haushey, Yahya Elshimali. (2023). Vaccination against SARS-CoV-2: Efficacy and Side effects, J. International Journal of Clinical Case Reports, 2(1) DOI: 10.31579/2834-8389/006
Copyright: © 2023 Rim M. Harfouch. This is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 25 January 2023 | Accepted: 03 February 2023 | Published: 13 February 2023
Keywords: COVID-19; vaccination; efficacy; side effects
Abstract
The COVID-19 pandemic has spread rapidly and infected about 509 million people with 6.22 million deaths all around the world. Given the lack of specific therapy for and the rapid spread of this virus, vaccination would be a significant way in the fight against the SARS-CoV-2 pandemic. On December 11, 2020 and December 18, 2020, respectively, the US Food and Drug Administration (FDA), granted emergency authorization to the Pfizer/BioN-Tech and Moderna COVID-19 vaccines. In this review, we summarize the development of COVID-19 vaccine technology and define the approved vaccines by FDA. We also brief the most common side effects and differences observed between available vaccines.
Introduction
Introduction:
The novel coronavirus SARS-COV-2 or COVID-19 was first found in Wuhan, China and is the cause of severe acute respiratory distress syndrome. Afterwards, this virus spread rapidly and became a global pandemic [1]. Although the fatality rate is low (reported to be 2.5% as of 12 February 2020), the accelerating transmission makes it a threat to mankind, and finding a curative treatment is a top priority. While no such treatment has been confirmed, many drugs and combinations are being suggested and some have even shown positive clinical results. On January 23, 2020, the first clinical trial for COVID-19 was registered, the number of trials then ascended to reach 125 registered trials by February 18, 2020 [2].
Common symptoms of Covid-19 include acute respiratory illness (cold-like disease), hyperthermia (fever>38º C), coughing, sore throat, and shortness of breath. Also, numerous sufferers may also experience digestive symptoms such as anorexia, diarrhea, and vomiting [3].
During the COVID-19 pandemic, people are facing major health care challenges, lockdowns, and stress, as there is no specific treatment and vaccination for this pandemic. Given the lack of specific therapy for and the rapid spread of this virus, vaccination would be a significant way in the fight against the SARS-CoV-2 pandemic [4,5].
Development of COVID-19 vaccines:
Pfizer-BioNTech vaccine (BNT162b2) is based on the mRNA technology to express the SARS-CoV-2 spike (S) gene and has shown a high efficacy rate against SARS-CoV-2 infection. Specifically, phase III trials showed that BNT162b2 has about 95(percentages) efficacy against laboratory-confirmed SARS-CoV-2 symptomatic infection, at least seven days after the second dose in the individual of 16 years and older without current or previous history of COVID-19. mRNA vaccines are a new type of vaccine that has been recently utilized. BNT162b2 mRNA vaccine has been developed to stimulate immune response against SARS-CoV-2 using mRNA coding SARS-CoV-2 spike protein. This vaccine was approved by the U.S. food and drug administration (FDA) on the 11th of December 2020 for EUA in individuals older than 16 years of age. On the other hand, two doses of Oxford-AstraZeneca adenovirus-vectored vaccine (ChAdOx1 nCoV-19) showed an overall 63(percentage) efficacy against symptomatic SARS-CoV-2 infection. This vaccine was authorized to be used in the age group of 18 years and older. Unlike BNT162b2, ChAdOx1 nCoV-19 uses replication-deficient chimpanzees adenovirus as a viral vector to express the SARS-COV-2 spike protein [6].
During 2020, 58 vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) be developed and in clinical trials, with some vaccines reportedly having more than 90(percentage) efficacy against COVID-19 in clinical trials. This remarkable achievement is much-needed good news as COVID-19 cases are currently at their highest daily levels globally. New vaccine efficacy results are reported now in The Lancet: investigators of four randomized, controlled trials conducted in the UK, South Africa, and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the Oxford–AstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 (AZD1222) in adults aged 18 years and older [7, 8].
On December 11, 2020 and December 18, 2020, respectively, the US Food and Drug Administration (FDA), granted emergency authorization to the Pfizer/BioN-Tech and Moderna COVID-19 vaccines. These two COVID-19 vaccines were developed quickly to benefit humanity and arrest the rise in the number of SARS-CoV-2 cases. From the time when the SARS-CoV-2 genome was released in early 2020 until these two vaccines received EUA status, less than one year passed. The fastest any vaccine had previously been developed, from viral sampling to approval, was four years, for mumps in the 1960s. There have been some concerns about potential adverse effects of these vaccines. The present study aims to highlight evidence about the pharmacological characteristics, indications, contraindications and adverse effects of Pfizer/BioNTech and Moderna vaccines [9].
The following table (1) summarize the FDA approved COVID-19 vaccines with details on shoots number, efficacy and side effects.
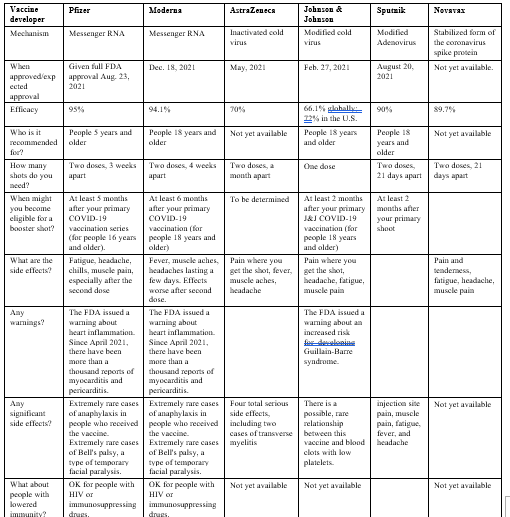
Side effects of covid-19 vaccines:
Clinical trials have shown that both Pfizer-BioNTech and Oxford-AstraZeneca vaccines were associated with various mild to moderate side effects, such as pain, redness or swelling at the site of injection, tiredness, headaches, chills, muscle, and joint aches, and fever.
According to a Saudi study, the participants who received the Oxford-AstraZeneca vaccine reported a significantly higher frequency of fatigue and headache than those who received the Pfizer-BioNTech vaccine [6].
Significant differences were observed between the side effect profiles of mRNA versus viral vector vaccines (predominantly Pfizer versus AstraZeneca). Overall, the recipients of mRNA vaccines reported a higher incidence of any self-reported side effects, which were, however, of significantly milder severity compared with those who received viral vector vaccines. While mRNA vaccines were associated with an increased incidence of local reactions, they were associated with a considerably lower incidence of systemic side effects including anaphylaxis, fever, swelling in the face or mouth or generalized swelling, flu-like illness, breathlessness and fatigue. Most importantly, mRNA vaccines were associated with a significantly lower incidence of severe side effects (requiring hospital care) [10].
On the other hand, another study conducted in Japan showed that individuals vaccinated with the mRNA-1273 vaccine were more likely to experience systemic reactions than those vaccinated with the BNT162b2 vaccine. Delayed injection site reaction was reported most frequently in middle-aged females after receiving the first dose of the mRNA-1273 vaccine [11].
The most common side effects of Sputnik V (adenovirus vector) are: injection site pain, fever, headache, fatigue, and muscle and joint pain. Moreover, unusual thrombotic cases have rarely been reported during the use of vector vaccines. The most important reasons involved in causing this rare complication are platelets and PF4 (platelet factor 4). The mechanisms that may be behind thrombotic thrombocytopenia after COVID-19 vaccination are: a) antibodies against PF4, b) the cross-reactivity of anti-spike antibodies and PF4, c) cross-reactivity of antibodies against adenovirus with PF4, d) interaction between spike protein and platelets, e) the direct interaction between adenoviral vector and platelets [12].
Conclusion
The efficacy of FDA-approved vaccines against COVID-19 ranged from 70-95% with the highest efficacy of Pfizer-BioNTech vaccine. mRNA based vaccines (Pfizer and Moderna) may have lower systematic side effects compared with vector based vaccines (Sputnik V and AstraZeneca), but mRNA vaccines have higher incidence of local reactions and may be associated with myocarditis and pericarditis according to thousand reports. Further studies on vaccine safety are recommended to strengthen public confidence in COVID-19 vaccines.
References
- Harfouch RM (2022). Antiviral Effects of Propolis against SARS-COV 2. Int J Clin Med Imaging 9:809
View at Publisher | View at Google Scholar - Harfouch, R.M., Alshaikh, S., Alshimaly, M., Assaad, A., Ahmad, J., Zoughaibi, H., Hammadi, M. and Elshimali, Y., (2021). Therapeutic approaches for covid 19: Challenges and successes. Annals of Clinical and Analytical Medicine, pp.228-233.
View at Publisher | View at Google Scholar - Jebur A , Harfouch T and Harfouch R. (2021) COVID-19 Pandemic in the Arab World: A Mini-Review Article. CPQ Medicine. 11(4), 01-10.
View at Publisher | View at Google Scholar - Harfouch RM. (2021) Cytokine Storm Syndrome in COVID-19 Patients: Characteristics and Diagnosis. Ann Clin Med Case Rep. 7(18):1-3.
View at Publisher | View at Google Scholar - Alhouri, Ali, et al.
View at Publisher | View at Google Scholar - Alhazmi A, Alamer E, Daws D, Hakami M, Darraj M, Abdelwahab S, Maghfuri A, (2021) Algaissi A. Evaluation of Side Effects Associated with COVID-19 Vaccines in Saudi Arabia. Vaccines (Basel). 18;9(6):674
View at Publisher | View at Google Scholar - Knoll MD, Wonodi C. (2021) Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 397(10269):72-74.
View at Publisher | View at Google Scholar - Al-Salam, J.A., Ibrahim, H., and Shoujaa, A. (2022)
View at Publisher | View at Google Scholar - Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. (2021) COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. Feb;25(3):1663-1669.
View at Publisher | View at Google Scholar - Mathioudakis AG, Ghrew M, Ustianowski A, Ahmad S, Borrow R, Papavasileiou LP, Petrakis D, Bakerly ND. (2021) Self-Reported Real-World Safety and Reactogenicity of COVID-19 Vaccines: A Vaccine Recipient Survey. Life (Basel). 17;11(3):249.
View at Publisher | View at Google Scholar - Kitagawa H, Kaiki Y, Sugiyama A, et al. (2022) Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan. J Infect Chemother. 28(4):576-581.
View at Publisher | View at Google Scholar - Zare H, Rezapour H, Mahmoodzadeh S, Fereidouni M. (2021) Prevalence of COVID-19 vaccines (Sputnik V, AZD-1222, and Covaxin) side effects among healthcare workers in Birjand city, Iran. Int Immunopharmacol. 101:108351.
View at Publisher | View at Google Scholar

 Clinic
Clinic
