Review Article | DOI: https://doi.org/10.31579/2834-5029/034
The Role of the Vaccine Ingredients Used in the Manufacture of Vaccine
- Ashwin Singh Chouhan *
Jai Narain Vyas University (New Campus), Jodhpur, Rajasthan, India.
*Corresponding Author: Ashwin Singh Chouhan, Jai Narain Vyas University (New Campus), Jodhpur, Rajasthan, India.
Citation: Ashwin Singh Chouhan. (2023), The Role of the Vaccine Ingredients Used in the Manufacture of Vaccine. International Journal of Biomed Research. 5(6): DOI:10.31579/2834-5029/034
Copyright: © 2023, Ashwin Singh Chouhan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 06 October 2023 | Accepted: 30 October 2023 | Published: 10 November 2023
Keywords: vaccine, manufacturing, technology, development, messenger rna, ingredients etc
Abstract
Vaccine development platform technologies that can produce a wide range of vaccines are emerging. Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global pandemic which has induced unprecedented ramifications, severely affecting our society due to the long incubation time, unpredictably high prevalence and lack of effective vaccines. Safe and effective vaccine against coronavirus disease (COVID)-19, pharmaceutical formulation science plays a critical role throughout the development, manufacturing, distribution, and vaccination phases DNA technology can simplify the complexity of manufacturing and facilitate consistent production of large quantities of antigen. Messenger RNA (mRNA)-based vaccines have shown promise against infectious diseases and several types of cancer in the last two decades.
Introduction
To stop the spread of future epidemics and meet infant vaccination demands in low- and middle-income countries, flexible, rapid and low-cost vaccine development and manufacturing technologies are required.
Vaccine development platform technologies that can produce a wide range of vaccines are emerging, including:
- Humanized, high-yield yeast recombinant protein vaccines
- Insect cell-baculovirus addomer vaccines
- Generalized modules for membrane antigens (gmma) vaccines
- RNA vaccines
Herein, existing and future platforms are assessed in terms of addressing challenges of scale, cost, and responsiveness.
To assess the risk and feasibility of the four emerging platforms, the following six metrics are applied:
- Technology readiness
- Technological complexity
- Ease of scale-up
- Flexibility for the manufacturing of a wide range of vaccines
- Thermostability of the vaccine product at tropical ambient temperatures
- Speed of response from threat identification to vaccine deployment
The assessment indicated that technologies in the order of increasing feasibility and decreasing risk are the yeast platform, ADDomer platform, followed by RNA and GMMA platforms. The comparative strengths and weaknesses of each technology are discussed in detail, illustrating the associated development and manufacturing needs and priorities. [1]
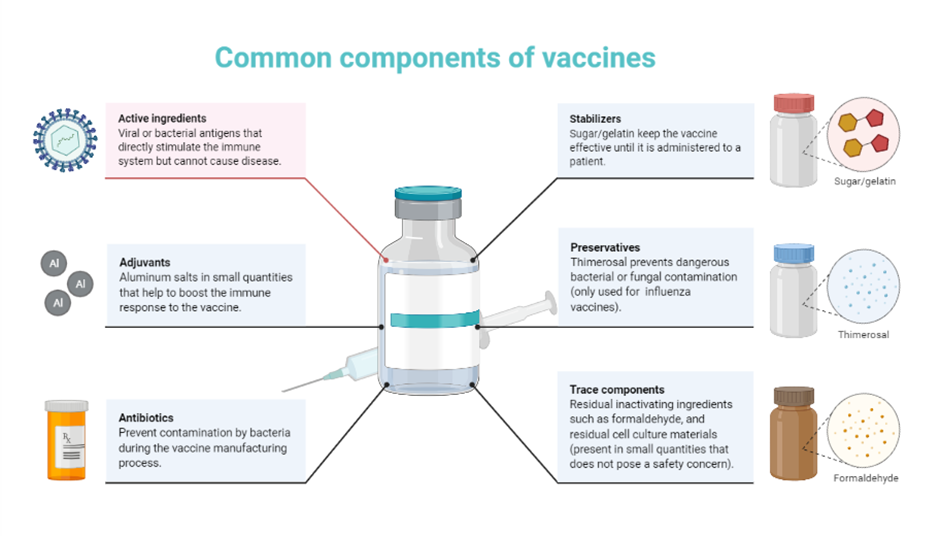
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global pandemic which has induced unprecedented ramifications, severely affecting our society due to the long incubation time, unpredictably high prevalence and lack of effective vaccines. One of the interesting notions is that there is an association between COVID-19 and cancer. Cancer patients seem to exhibit exacerbated conditions and a higher mortality rate when exposed to the virus. Therefore, vaccines are the promising solution to minimise the problem amongst cancer patients threatened by the new viral strains. However, there are still limitations to be considered, including the efficacy of COVID vaccines for immunocompromised individuals, possible interactions between the vaccine and cancer, and personalised medicine. Not only to eradicate the pandemic, but also to make it more effective for immunocompromised patients who are suffering from cancer, a successful vaccine platform is required through the implementation of nanotechnology which can also enable scalable manufacturing and worldwide distribution along with its faster and precise delivery. In this review, we summarise the current understanding of COVID-19 with clinical perspectives, highlighting the association between COVID-19 and cancer, followed by a vaccine development for this association using nanotechnology. We suggest different administration methods for the COVID-19 vaccine formulation options. This study will contribute to paving the way towards the prevention and treatment of COVID-19, especially for the immunocompromised individuals. [2] In the race for a safe and effective vaccine against coronavirus disease (COVID)-19, pharmaceutical formulation science plays a critical role throughout the development, manufacturing, distribution, and vaccination phases. The proper choice of the type of vaccine, carrier or vector, adjuvant, excipients, dosage form, and route of administration can directly impact not only the immune responses induced and the resultant efficacy against COVID-19, but also the logistics of manufacturing, storing and distributing the vaccine, and mass vaccination. In this review, we described the COVID-19 vaccines that are currently tested in clinical trials and provided in-depth insight into the various types of vaccines, their compositions, advantages, and potential limitations. We also addressed how challenges in vaccine distribution and administration may be alleviated by applying vaccine-stabilization strategies and the use of specific mucosal immune response-inducing, non-invasive routes of administration, which must be considered early in the development process. [3]
mRNA vaccines represent a promising alternative to conventional vaccine approaches because of their high potency, capacity for rapid development and potential for low-cost manufacture and safe administration. However, their application has until recently been restricted by the instability and inefficient in vivo delivery of mRNA. Recent technological advances have now largely overcome these issues, and multiple mRNA vaccine platforms against infectious diseases and several types of cancer have demonstrated encouraging results in both animal models and humans. This Review provides a detailed overview of mRNA vaccines and considers future directions and challenges in advancing this promising vaccine platform to widespread therapeutic use. [4] Enabled by new approaches for rapid identification and selection of human monoclonal antibodies, atomic-level structural information for viral surface proteins, and capacity for precision engineering of protein immunogens and self-assembling nanoparticles, a new era of antigen design and display options has evolved. While HIV-1 vaccine development has been a driving force behind these technologies and concepts, clinical proof-of-concept for structure-based vaccine design may first be achieved for respiratory syncytial virus (RSV), where conformation-dependent access to neutralization-sensitive epitopes on the fusion glycoprotein determines the capacity to induce potent neutralizing activity. Success with RSV has motivated structure-based stabilization of other class I viral fusion proteins for use as immunogens and demonstrated the importance of structural information for developing vaccines against other viral pathogens, particularly difficult targets that have resisted prior vaccine development efforts. Solving viral surface protein structures also supports rapid vaccine antigen design and application of platform manufacturing approaches for emerging pathogens. [5]
DNA or mRNA vaccines have potential advantages over conventional vaccines since they are easier to manufacture and have higher safety profiles. In particular, self-amplifying RNA (saRNA) derived from alphavirus expression vectors has shown to be very efficient to induce humoral and cellular responses against many antigens in preclinical models, being superior to non-replicating mRNA and DNA. This is mainly due to the fact that saRNA can provide very high expression levels and simultaneously induces strong innate responses, potentiating immunity. saRNA can be administered as viral particles or DNA, but direct delivery as RNA represents a safer and more simple approach. Although saRNA can be delivered as naked RNA, in vivo transfection can be enhanced by electroporation or by complexing it with cationic lipids or polymers. Alphavirus saRNA could have broad application to vaccinate against human pathogens, including emerging ones like SARS-CoV-2, for which clinical trials have been recently initiated. [6] One of the earliest methods used in the manufacture of stable and safe vaccines is the use of chemical and physical treatments to produce inactivated forms of pathogens. Although these types of vaccines have been successful in eliciting specific humoral immune responses to pathogen-associated immunogens, there is a large demand for the development of fast, safe, and effective vaccine manufacturing strategies. Radiation sterilization has been used to develop a variety of vaccine types, because it can eradicate chemical contaminants and penetrate pathogens to destroy nucleic acids without damaging the pathogen surface antigens. Nevertheless, irradiated vaccines have not widely been used at an industrial level because of difficulties obtaining the necessary equipment. Recent successful clinical trials of irradiated vaccines against pathogens and tumors have led to a reevaluation of radiation technology as an alternative method to produce vaccines. In the present article, we review the challenges associated with creating irradiated vaccines and discuss potential strategies for developing vaccines using radiation technology. [7] Improved understanding of antigenic components and their interaction with the immune system, as supported by computational tools, permits a sophisticated approach to modern vaccine design. Vaccine platforms provide an effective tool by which strategically designed peptide and protein antigens are modularized to enhance their immunogenicity. These modular vaccine platforms can overcome issues faced by traditional vaccine manufacturing and have the potential to generate safe vaccines, rapidly and at a low cost. This review introduces two promising platforms based on virus-like particle and liposome, and discusses the methodologies and challenges. [8] Messenger RNA (mRNA)-based vaccines have shown promise against infectious diseases and several types of cancer in the last two decades. Their promise can be attributed to their safety profiles, high potency, and ability to be rapidly and affordably manufactured. Now, many RNA-based vaccines are being evaluated in clinical trials as prophylactic and therapeutic vaccines. However, until recently, their development has been limited by their instability and inefficient in vivo transfection. The nanodelivery system plays a dual function in RNA-based vaccination by acting as a carrier system and as an adjuvant. That is due to its similarity to microorganisms structurally and size-wise; the nanodelivery system can augment the response by the immune system via simulating the natural infection process. Nanodelivery systems allow non-invasive mucosal administration, targeted immune cell delivery, and controlled delivery, reducing the need for multiple administrations. They also allow co-encapsulating with immunostimulators to improve the overall adjuvant capacity. The aim of this review is to discuss the recent developments and applications of biodegradable nanodelivery systems that improve RNA-based vaccine delivery and enhance the immunological response against targeted diseases. [9]
In the 21st century, an array of microbiological and molecular allow antigens for new vaccines to be specifically identified, designed, produced and delivered with the aim of optimising the induction of a protective immune response against a well-defined immunogen. New knowledge about the functioning of the immune system and host pathogen interactions has stimulated the rational design of vaccines. The design toolbox includes vaccines made from whole pathogens, protein subunits, polysaccharides, and pathogen-like particles, use of viral/bacterial vectors, plus adjuvants and conjugation technology to increase and broaden the immune response. Processes such as recombinant DNA technology can simplify the complexity of manufacturing and facilitate consistent production of large quantities of antigen. Any new vaccine development is greatly enhanced by, and requires integration of information concerning:
1. Pathogen life-cycle & epidemiology: Knowledge of pathogen structure, route of entry, interaction with cellular receptors, subsequent replication sites and disease-causing mechanisms are all important to identify antigens suitable for disease prevention. The demographics of infection, specific risk groups and age-specific infection rates determine which population to immunise, and at what age.
2. Immune control & escape: Interactions between the host and pathogen are explored, with determination of the relative importance of antibodies, T-cells of different types and innate immunity, immune escape strategies during infection, and possible immune correlates of protection. This information guides identification and selection of antigen and the specific immune response required for protection.
3. Antigen selection & vaccine formulation: The selected antigen is formulated to remain suitably immunogenic and stable over time, induce an immune response that is likely to be protective, plus be amenable to eventual scale-up to commercial production.
4. Vaccine preclinical & clinical testing: The candidate vaccine must be tested for immunogenicity, safety and efficacy in preclinical and appropriately designed clinical trials. This review considers these processes using examples of differing pathogenic challenges, including human papillomavirus, malaria, and ebola. [10]
Method and material: We conducted this research paper by observing the different types of reviews, as well as conducting and evaluating literature review papers.
Result and Discussion
In this research, we found that how challenges in vaccine distribution and administration may be alleviated by applying vaccine-stabilization strategies and the use of specific mucosal immune response-inducing, non-invasive routes of administration, which must be considered early in the development process. Recent technological advances have now largely overcome these issues, and multiple mRNA vaccine platforms against infectious diseases and several types of cancer have demonstrated encouraging results in both animal models and humans. Solving viral surface protein structures also supports rapid vaccine antigen design and application of platform manufacturing approaches for emerging pathogens. In particular, self-amplifying RNA (saRNA) derived from alphavirus expression vectors has shown to be very efficient to induce humoral and cellular responses against many antigens in preclinical models, being superior to non-replicating mRNA and DNA. Alphavirus saRNA could have broad application to vaccinate against human pathogens, including emerging ones like SARS-CoV-2, for which clinical trials have been recently initiated. Although these types of vaccines have been successful in eliciting specific humoral immune responses to pathogen-associated immunogens, there is a large demand for the development of fast, safe, and effective vaccine manufacturing strategies. In the present article, we review the challenges associated with creating irradiated vaccines and discuss potential strategies for developing vaccines using radiation technology. In the 21st century, an array of microbiological and molecular allow antigens for new vaccines to be specifically identified, designed, produced and delivered with the aim of optimising the induction of a protective immune response against a well-defined immunogen. Top of Form Bottom of Form.
Conclusion
In our research, we concluded that challenges in vaccine distribution and administration may be alleviated by applying vaccine-stabilization strategies and the use of specific mucosal immune response-inducing, non-invasive routes of administration, which must be considered early in the development process. Although these types of vaccines have been successful in eliciting specific humoral immune responses to pathogen-associated immunogens, there is a large demand for the development of fast, safe, and effective vaccine manufacturing strategies.
Acknowledgment:
We grateful thanks to all the sincere and extremely helpful friends for their support and help for the completion of work. Last but not the least, we thankful to all those who cooperated and helped me directly or indirectly to carry out this work.
Ethical Approval:
Ethical approval was not required for this letter. All data used is publicly accessible.
Funding:
There were no external sources of funding for this research.
Data Availability Statement:
We do not wish to share our data before we have thoroughly analyzed. All data sources described in the study are directed at the corresponding author.
Financial Support and Sponsorship:
Nil.
Conflicts of Interest:
All authors are declaring that they have no conflicts of interest.
Authors’ Contribution
The first author developed the proposal, and then collected and analyzed the data under supervision of respective advisers. The rest of authors undertook the literature search and review, and gave constructive comments and guidance to work with the main author with respect to the research objective.
References
- Zoltán Kis , et al. (2019). Biotechnology Systems & Synthetic Biology.Nanobiotic.Medicine Journal; 14(7):1-2
View at Publisher | View at Google Scholar - Hyun Jee Han, et al. (2021). journal of International Immunopharmacology; 90: 107247
View at Publisher | View at Google Scholar - Jieliang Wang et al. (2020). AAPS PharmSciTech. 21(6):225.
View at Publisher | View at Google Scholar - Norbert Pardi et al.(2018). Nat Rev Drug Discov. 17(4):261-279.
View at Publisher | View at Google Scholar - Barney S Graham et al.(2019). Annu Rev Med. 70:91-104
View at Publisher | View at Google Scholar - María Cristina Ballesteros-Briones et al. (2020). Curr Opin Virol.44:145-153.
View at Publisher | View at Google Scholar - Ho Seong Seo. (2015). Clin Exp Vaccine Res. 4(2):145-58.
View at Publisher | View at Google Scholar - Hayley K Charlton Hume, Linda H L Lua. (2017). Vaccine. 35(35 Pt A):4480-4485.
View at Publisher | View at Google Scholar - Iman M Alfagih et al. (2020). Pharmaceutics. 13(1):45.
View at Publisher | View at Google Scholar - Anthony L Cunningham et al., (2016). Vaccine. 34(52):655-666.
View at Publisher | View at Google Scholar

 Clinic
Clinic
