Research Article | DOI: https://doi.org/10.31579/2834-8508/002
Synthesis of dibenzoylmethane-flavonoid hybrids as potential uv filters. Hybrids of chalcones
1Department of Organic Chemistry, University of the Republic, Montevideo, Uruguay;
2DQIAQF, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, Argentina.
*Corresponding Author: Federico Svarc, DQIAQF, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, Argentina.
Citation: Gabriel Sagrega, Federico Svarc, Yanina Minaberry. (2022) Synthesis of Dibenzoylmethane-Flavonoid Hybrids as Potential UV Filters. Hybrids of Chalcones. Archives of Clinical and Experimental Pathology.1(1); Doi:10.31579/2834-8508/002
Copyright: ©2022 Federico Svarc, This is an open-access article distributed under the terms of The Creative Commons. Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 12 September 2022 | Accepted: 19 September 2022 | Published: 26 September 2022
Keywords: uv filters; dibenzoylmethanes; chalcones; flavonoid hybrids; photostability
Abstract
Background: It has been reported that chalcones (a family of flavonoids) have activity as UV rays absorbers. Previously we have reported that the compounds2´-hydroxy-4- methoxychalcone and 2´-hydroxy-4-methoxy dibenzoylmethane have the basic properties of UVA filters (both show their maximum absorption in the range 350-370 nm with good photostability)
Objective: in this work, we describea new series of chalcone-dibenzoylmethane hybrid candidates as synthetic UV filtersand flavonoids
Method: The compounds resultedfrom multi step synthesisstarting from 4-formylbenzoic acid and 2´- hydroxy acetophenone. Purification was done by recrystallization or column chromatography.
Results: The compounds were obtained with good yields and characterized by spectroscopy.
Conclusion: They are good candidates for UVA filters in cosmetics. Drs. Svarc and Minaberry will performthe corresponding photostability studies.
Introduction
Originally, the term “flavonoid” comes from the fact it is structurally related to flavane (2 – phenylcromane) (Figure 1) [1-4].
More correctly, they should be considered as derivatives of 1,3-diphenyl propane (C6- C3-C6 skeleton). Other compounds that are structurally related to flavonoids are the derivatives of 1,2-diphenyl propane(isoflavonoids) and 1,1-diphenyl propane (neoflavonoids).
In all of the three cases, fragment C3 can take part in a closed ring or stay as an open chain.
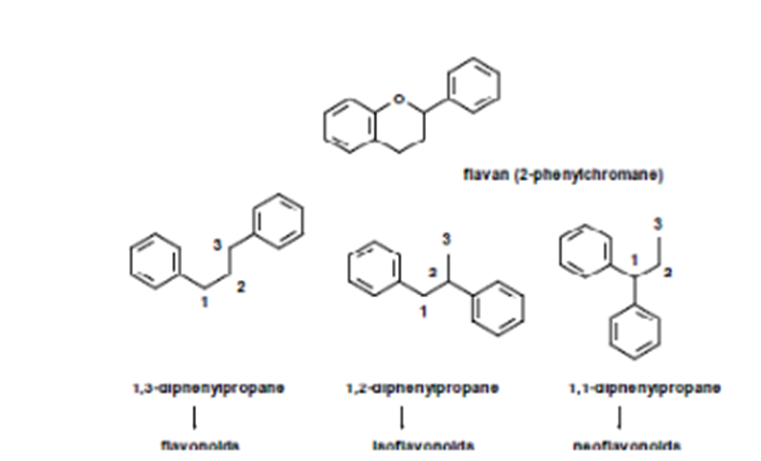
Flavonoids possess the same basic skeleton. The different types and families differ structurally because of their substituent pattern and oxidation level of C in the C3 fragments (or as a part of a heterocycle), while different compounds within a family differ inthe substitution patternof the rings A and B. Figure 2.
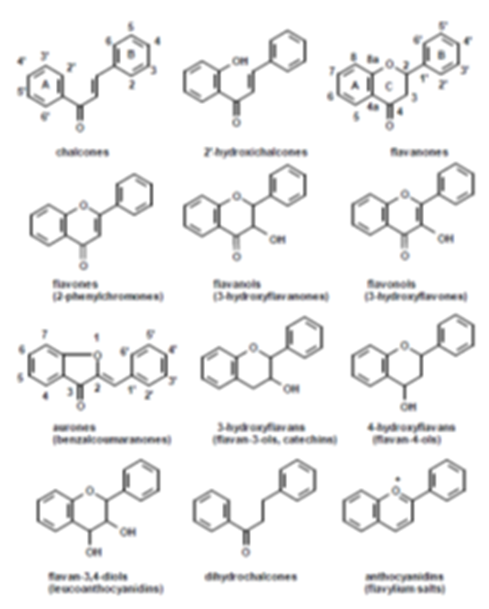
Flavonoids are widely distributed in nature, mostly in the vegetal kingdom, and their range of biological activities is large. They are one of the most important secondarymetabolites of plants with structural and functional capabilities. Many natural and synthetic flavonoids have some type of biological activity in animals and men, being used in the industry too.
Flavonoids are found everywhere in the vegetal kingdom, almost in every terrestrial plant (superior and inferior), and also in some algae [3]. Around 2000 more than 8000 individual compounds [5] were known. They play different roles in the plant´s ecology, acting as antioxidants, antimicrobials, photoreceptors, UV protectants, visual attractors, chemical attractors, or repellents and inductors of nodulation in bacteria fixing Nitrogen. They also act as protectants from oxidative stress, trapping reactive oxygen species (ROS) produced during photosynthesis. Due to their capacity to absorb UV radiation from the sun, they protect the plants from damage caused and trap the ROS generated [6].
Flavonoids have a wide range of biological activities[7], within them as antioxidants, antimicrobials (including bacteria, fungi, viruses, plasmoids), anti-tumoral, chimo, and cytotoxic-preventive. Also possess anti-allergic, anti inflammatory, hypotensive, vasodilator, gastroprotection, anti diabetes properties and help to fight against capillaryfragility and platelet aggregation.
As for other natural compounds, the bioactivity of flavonoids depends on the nature, number, and position of the substituents on their skeleton [8,9]. Frequently the biological activity is related to free hydroxyl moieties. As an example, the antioxidant capacity increaseswith the number of hydroxiles. It is widely acceptedthat fruits and vegetals have many healthyproperties. People who consume a good quantityof those have a healthystyle of life, which is important in face of chronic diseases. We think that a supplement of antioxidants (particularly flavonoids) by daily ingesta of food gives additional protection against in vivo oxidation of biomolecules in the cells. Studies in vitro and on animals have been made to prove this hypothesis. There exists enough epidemiological evidenceshowing a correlation between individuals that keep a diet rich in fresh fruits and vegetals with benefit on health. They contribute to avoiding degenerative processes, diminishing particularly incidenceand mortality from cancer, heart disease, and brain vascularization [10-14].
It has been reported that some of the chalcones are capable of the negative effects of UV radiation. Their protective activityagainst the sun is generatedby a high UV absorbance in the region UVB-UVA[15], corresponding to the electronic transition n and *of HOMO and LUMO energy levels in the conjugated electronic system of these molecules. By the introduction of electron donor substituents (bathochromic change) or acceptor substituents (hypsochromic change) in both rings o the UV absorbing range of chalcones can be displaced, being the major change on ring A [16] (Figure 2).One of the drawbacks to using chalconesas solar filtersis their relatively low photostability and their photodecomposition products [17-19].
Many chalcones show protective action on deleterious effects produced by UV rays, as trans-chalcone, buteine, monospermoside, lico-chalcone A, florentin, and hesperidin derivatives. Non-substituted trans-chalcones did not show in rats any potential activity towards inflammation and oxidative stress. A formulation with 1% of non-substituted trans-chalcones can protect the skin from UVB radiationby reducing the level of the alfa tumoral necrosis factor (TNF- and by improving the detoxification and antioxidant systemsincreasing haemo-oxygenase 1 (HO1)[18,19].Systemic administration of trans-chalcone seems to inhibitUV-induced skin inflammation and prevent oxidativestress targeting the nicotinamide adeninedinucleotide phosphate H (NADPH) oxidase and cytokine generation [18].
In vitro and in vivo studies showed that the licochalcone A has a huge protectant effect against oxidative stress and UVB-induced inflammation. It inhibits prostaglandin E2, cyclooxygenase (COX-2), lipoxygenase, and the nuclear factor kappa enhancer belonging to the light chain of B activated cells (NF-B) and Nrf2 [20-22].
An in vivo study has shown that a topicalformulation of licochalcone A reduced in a significant wayerythema, irritation, and oxidative processes induced by UV rays on the skin [21-23].
Hesperidin methyl chalcone and related compounds also exhibit a high degree of protection in the UV [24].
The topical and systemic administration of hesperidin-methyl chalcone on mice inhibits the oxidative stress induced by UVB rays by reduction of ROS and other free radicals, improves endogen antioxidants, and inhibits inflammation by cytokine suppression [25].
Chalcones have maximum absorption of around 350 nm, thus they are useful as UV absorbents and can be incorporated into paints, plastic materials, synthetic fibers, and cosmetics [27-30].
Lahorkar has reported that some chalcones (butein, monospermoside) increase Avobenzone stability.
Our research group has reported that a 2-OH group in the A ring (Figure 2) of a chalcone or dibenzoyl methane increases the photostability of the molecules. In this sense, 2-hydroxy-4-methoxy-chalcone and 2´-hydroxy-4-methoxy-dibenzoylmethane present a good photostability and are candidates to be used as UVA filters in cosmetic formulations [32].
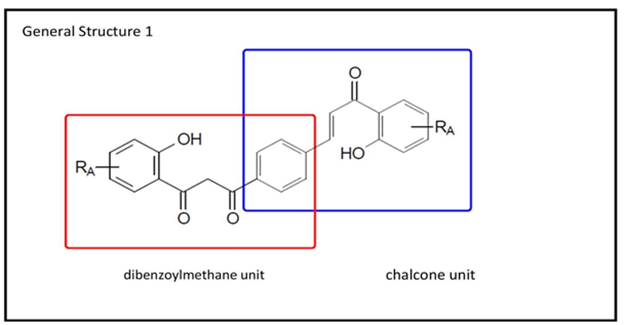
As a consequence of a research line related to the synthesis of dibenzoylmethanes and flavonoids, this work describesthe preparation of a small library of chalcone-dibenzoylmethane hybrids, for their subsequent evaluation as sunscreens. Their general structure1 is shown in Figure 3.
Materials And Methods
All compounds were prepared by typical organic chemistry reactions (aldol condensation, oxidative cyclization, acid chloride formation, esterification, and Baker-Venkataraman rearrangement) using previously optimized conditions reported by us [33,34]. Some reactions were carried out in a nitrogen atmosphere. All reactions were monitored by thin-layer chromatography (TLC). The compounds were isolated by common procedures (extraction with organic solvents, washing with brine, drying of the organic layers, and concentration in vacuo), and purified by recrystallization or column chromatography (CC). The compounds were characterized by proton and carbon nuclear magnetic resonance spectroscopy (1H-NMR and 13C-NMR) and mass spectrometry (MS).
All reagents were purchased from commercial sources and used without purification unless otherwise indicated. All solvents were dried and distilled before use. AcOEt and hexanes were dried over MgSO4 and distilled. THF was treated with CaH2 to remove peroxides, and dried by refluxing with benzophenone over Na wire until a blue color persisted, then distilled and collected. CH2Cl2 was dried by refluxing over P2O5 for 3 h, then distilled and collected. Pyridine was dried by heating under reflux over anhydrous KOH and then distilled. All reactions were monitored by TLC on polyester plates coated with Alugram Sil G/UV254 using various solvent systems. Column chromatography was carried out on silica gel (Merck, 60–230 mesh) using hexanes as the initial eluent, followed by a suitable solvent gradient. 1H and 13C NMR spectra were recordedat 30 °C on a Bruker DPX-400spectrometer at 400 MHz and 100 MHz, respectively.
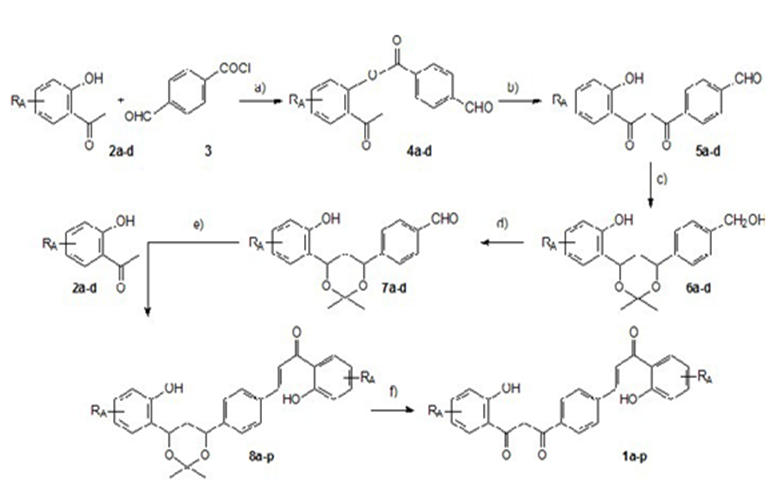
First, acetophenonesnot available in our laboratory were synthesized. 2'-hydroxy-4'- butyloxyacetophenone was prepared from 2', 4'-dihydroxyacetophenone and 2'-hydroxy 4',6'- dimethoxy acetophenone from phloroglucinol. The hybrid compounds were prepared in several steps,using previously optimized conditions [33,34] (Figure 4):
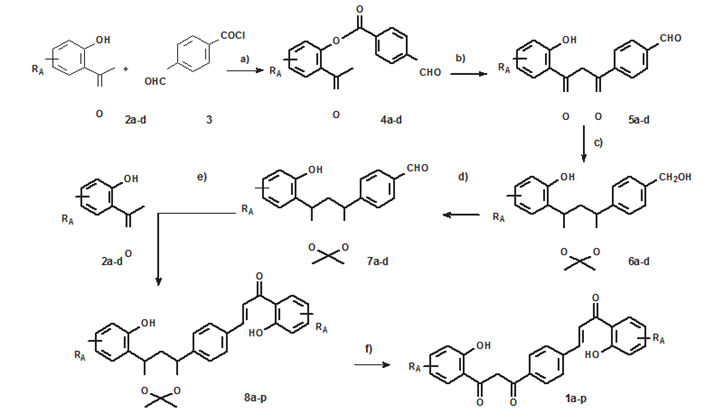
Experimental
- synthesis of esters from 4-formylbenzoic acid and acetophenones 2a-d. First, the chloride of 4-formylbenzoic acid is prepared by treating the acid with SOCl2 in CH2Cl2. The acid chloride isnot purified and was reacted with acetophenones
2a-d to form esters 4a-d
- Baker-Venkataraman rearrangement to form dibenzoylmethanes 5a-d
Subsequently, carbonyl groups of the dibenzoylmethanes were protected to prepare the chalcones by aldolcondensation.
This is done in 3 steps:
c1) reduction of carbonyl groups
c2) formation of an acetonide with the hydroxyl groups in position1,3
- mild oxidation of the CH2OH group to regenerate the aldehydegroup
- aldol condensation with acetophenone 2a-d to form hybrid compound a-p
- f1) removal of the acetonide group
f2) mild oxidation of 1,3 hydroxyl groups to regenerate dibenzoylmethane RA = H, 4'-OMe, 4'-OBu, 4',6'-diOMe
Synthesis of 4-formylbenzoic acid chloride
SOCl2 (20 mmol) and 3 drops of DMF (catalyst) were added to a suspension of 4-formylbenzoic acid (10 mmol) in CH2Cl2 (10 mL). The reaction mixture is refluxed for 3 h. The reaction mixture is allowed to cool to r.t. and the solvent was evaporated under reduced pressure. The product was not purifiedand is used directly in the next step.
General method 1: Synthesis of 2'-hydroxy acetophenone esters
A solution of the corresponding 2'-hydroxy acetophenone (10 mmol) and the acid chloride (10 mmol) in anhydrous pyridine (15 mL) was stirred at r.t. for 2 h. After this time, the mixture was neutralized with 10% HCl and extracted with AcOEt (3 X 50 mL). The combined organic layers were washed with water until neutrality (3 x 50 mL), with saturated NaCl solution (50 mL), and dried over anhydrous MgSO4. The solvent was evaporated under reduced pressure and the product was purified by CC.
General method 2: Synthesis of dibenzoylmethanes (Baker-Venkataraman rearrangement)
To a solution of the corresponding 2'-hydroxy acetophenone ester (1 mmol) in anhydrous pyridine (5 mL), KOH (850 mg, 15 mmol) was added. The reaction mixture was heated to 60 °C for 30 minutes. After this time the mixture was neutralized with 10% HCl and extracted with AcOEt (3 X 50 mL). The combined organic layers were washed with water until neutrality (3 x 50 mL), with saturated NaCl solution (50 mL), and dried over anhydrous MgSO4. The solvent was evaporated under reduced pressure and the product was purified by CC.
General method 3. Reduction of carbonyl groups
To a solution of the corresponding dibenzoylmethane (2 mmol) in absolute EtOH (40 mL) at 0°C, NaBH4 (80 mg, 2.1 mmol) was added.The suspension was stirred at 0 °C for 8 h. The reaction mixture was poured into H2O (100 mL) and stirred until the evolution of H2 ceases, it was carefully neutralized with a 10% HCl solution and extracted with AcOEt (3 x 50 mL). The combined organic layers were washed with water until neutrality, with saturated NaCl solution (50mL), and dried over anhydrous MgSO4. The solventwas evaporated under reduced pressureand the product was purified by CC.
General method 4. Formation of acetonides
To a solution of 1,3 diol (1 mmol) in anhydrous acetone(10 mL), and under N2 atmosphere withvigorous stirring anhydrous, p-TsOH (0.1 mmol) was added. The reaction mixture was refluxed for 5 h. The solvent was evaporated under reduced pressure and the residue was dissolved in AcOEt (50 mL). The organic layer was washed with water until neutrality (3 x 20 mL), with saturated NaCl solution (20 mL), and dried over anhydrous MgSO4. The solvent was evaporated under reduced pressure and the product was purified by CC.
General method5. Oxidation of the -CH2OH group
To a solution of acetonide (1 mmol) in CH2Cl2 (10 mL), under N2 atmosphere and with vigorous stirring, PCC/alumina (25% by weight) was added. The reaction mixture was stirred at r.t. for 2h. The solvent was evaporated under reduced pressure and the residue was dissolved in AcOEt (50 mL). The organic layer was washed with water until neutrality (3 x 20 mL), with saturated NaCl solution (20 mL), and dried over anhydrous MgSO4. The solvent was evaporated under reduced pressure and the product was purified by CC
General method 6. Synthesis of 2'-hydroxy chalcones
To a solution of the corresponding 2’-hydroxy acetophenone (10 mmol) in anhydrous THF (25 mL), NaH (1.2 g dispersion in mineral oil at 50%, 25 mmol), was added in portions, under N2 atmosphere, and with vigorous stirring. When the evolution of H2 ceased, a solution of the corresponding benzaldehyde (10 mmol) in anhydrous THF (25 mL) was added dropwise over 15 min and the mixture was stirred at r.t. for 16 h. The mixturewas poured cautiously over ice water (50 mL) to destroy excess NaH and stirred until the evolution of H2 ceased. The mixture was acidified with 25% HCl and extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with H2O (3 × 50 mL) and brine (50 mL) and dried (anhydrous MgSO4). The soln was concentrated under vacuum at 40 °C until it reached one-third of its original volume. The solution was cooled to 0 °C for 12 h to give a crystalline product which was separated by filtration. The 2’-hydroxy chalconesobtained in this way are sufficiently pure for most purposes.
General method 7. Deprotection of alcoholic groups
To a solutionof the corresponding acetonide (1 mmol) in MeOH-water (1:1 v/v; 20 mL), anhydrousp-TsOH (10mg, 0.058 mmol) was added. The reaction mixture was stirred for 2 h at r.t. and then the solvent was evaporated under reduced pressure. After this, water (50 mL) was added and the mixturewas extracted with AcOEt (3 x 50 mL). The combined organiclayers were washed with water until neutrality (3 x 50 mL),with saturated NaCl solution (50 mL), and dried over anhydrous MgSO4. The solvent was evaporated under reduced pressure and the product was purified by CC.
General method 8. Oxidation of alcoholic groups (1,3-diols)
To a solution of the corresponding diol (1 mmol) in CH2Cl2 (10 mL), under N2 atmosphere and with vigorous stirring, PCC/alumina (25% by weight) was added. The reaction mixture was stirred at r.t. for 2 h. The solvent was evaporated under reduced pressure, and the residue was dissolvedin AcOEt (50 mL). The organic layerwas washed with water until neutrality (3 x 20 mL), with saturated NaCl solution (20 mL), and dried over anhydrous MgSO4. The solvent was evaporated under reducedpressure and the product was purified by CC.
Results
Sixteen compounds were prepared from readily available reagents with 18-27% overall yields and a fair degree of purity. All compounds were characterized spectroscopically by NMR and MS.
Discussion
There is still an international need to develop broad spectrum sunscreen productswith an adequate UVB/UVA balance, while the approvedfilters available in the UVA are scarce. Currently, one of the few UVA filtersapproved in the United States and Europe is tert-butylmethoxydibenzoylmethane (BMDM, avobenzone). However, this compound is unstable from a photochemical pointof view and cannot be used in combination with certain sunscreens [32].
Recent studies show that the irradiation of avobenzone causes a breakdown of the molecule in radicals, which generates compounds, such as arylglyoxals and benzyls [35], or react with other sunscreens [36].
Studies of the biological properties of photodecomposition products indicate that arylglyoxals are strong photosensitizers. They are also very electrophilic and react quickly with the arginine of proteins. On the other hand, benzylsare cytotoxic [37].
Different ways to photostabilize avobenzone have been reported. Among them are UV pearls (encapsulated sunscreens), microspheres (hollow spheres of styrene-acrylate copolymers), ROS (reactive oxygen species) trappers, and inhibitors of the triplet-triplet and singlet-singlet mechanism [38].
None of these proposals seem to have been successful. The solution should not be to use photochemically unstable molecules that require additional molecules to make them stable in a cosmetic formulation. [32].
Considering this background, and due to the growingdemand for sunscreen, the discovery of new, safer, and more effective compounds is of great importance. In this paper, we described the synthesis of a set of dibenzoylmethanes and chalcones hybrids as potentially promising UV filters.
Continuing previous work [32] Drs. Svarc and Minaberry will test these compounds for their photostability at Buenos Aires University.
Conclusion
Sixteen compoundswere prepared from readily availablereagents in good yields. All compounds were characterized spectroscopically by NMR and MS. Since these techniques are simple, they are suitable for the preparation of a large number of compounds in a convenient quantityand degree of purity. In a later stage, the determination of UV parameters and photodegradation studies will be carried out.
List Of Abbreviations
UV: ultraviolet
NMR: nuclear magneticresonance
1H-NMR and: proton nuclear magnetic resonance 13C-NMR: 13-carbon nuclear magnetic resonance
MS: mass spectrometry
Py: pyridine
p-TsOH: para-toluene sulfonic acid TLC: thin layer chromatography CC: column chromatography AcOEt: ethyl acetate
PCC: pyridinium chlorochromate THF: tetrahydrofuran
DMF: dimethylformamide
Conflict Of Interest: The authors declareno conflicts of interest.
Acknowledgments: We thank the UDELAR (Uruguay) that allowed and financed the work of Gabriel Sagrera andINQUIMAE (CONICET—UBA) where all the photochemical measurements are done.
References
- Markham, K. R. Mabry, T. J. (1975). The Flavonoids. Ed. Chapman and Hall London.
View at Publisher | View at Google Scholar - Mabry, T. J. Markham, K. R. Thomas, M. B. (1970). The Systematic Identification of Flavonoids. Springer-Verlag, Heidelberg.
View at Publisher | View at Google Scholar - Mabry, T. J. Ulubelen, A. (1980). Chemistry, and utilization of phenylpropanoids including flavonoids, coumarins, and lignans. J. Agr. Food Chem. 28:188-196.
View at Publisher | View at Google Scholar - Geissman, T. A. (1962). The Chemistry of Flavonoid Compounds. MacMillan, New York.
View at Publisher | View at Google Scholar - Pietta, P.-G. (2000). Flavonoids as antioxidants. J. Nat. Prod. 63:1035-1042.
View at Publisher | View at Google Scholar - Stapleton, A. E. Walbot, V. (1999). Flavonoids can protect maize DNA from the induction of ultraviolet radiation damage. Plant Physiol. 105:881-889.
View at Publisher | View at Google Scholar - Di Carlo, G. Mascolo, N. Izzo, A. A. Capasso, F. (1999). Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 65:337-353
View at Publisher | View at Google Scholar - Hernandez Galan, R. Massanet, G. M. Rodriguez Luis, F. Salva, J. (1990). 3- Isoprenylcoumarins. Phytochemistry .29:2053-2059.
View at Publisher | View at Google Scholar - Chen, Z. Y. Chan, P. T. Ho, K. Y. Fung, K. P. Wang, J. (1996). Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem. Phys. Lipids. 79:157-163.
View at Publisher | View at Google Scholar - Hertog, M. G. Feskens, E. J. Hollman, P. C. Katan, M. B. Kromhout, D. (1993). Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 342:1007-1011.
View at Publisher | View at Google Scholar - Hertog, M. G. L.Kromhout, D. Aravanis, C. Blackburn, H. Buzina, R. et al. (1995).Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 155:381-386.
View at Publisher | View at Google Scholar - Hollman, P. C. H. Katan, M. B. (1997). Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 51:305-310.
View at Publisher | View at Google Scholar - Ingram, D. Sanders, K. Kolybaba, M. Lopez, M. (1997). Case-control study of phytoestrogens and breast cancer. Lancet 350:990-994.
View at Publisher | View at Google Scholar - Yochum, L. Kushi, L. H. Meyer, K. Folsom, A. R. (1999). Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am. J. Epidemiol.
View at Publisher | View at Google Scholar - Xue, Y. Mou, J. Liu, Y. Gong, X. Yang, Y. An, L. (2010). An ab initio simulation of the UV/visible spectra of substituted chalcones. Centr. Eur. J. Chem. 8(4):928-936
View at Publisher | View at Google Scholar - Shin, D. Song, D. Jung, K. Moon J. (2001). Photochemical transformation of chalcone derivatives. J. Photosci. 8(1):9-12
View at Publisher | View at Google Scholar - Sisa, M. Bonnet, S. L. Ferreira, D. Van Der Westhuizen, J. H. (2010). Photochemistry of flavonoids. Molecules. 15(8): 5196-5245
View at Publisher | View at Google Scholar - Kostyuk, V. Potapovich, A. Albuhaydar, A. R. Mayer, W. De Luca, C. Korkina, L. (2018). Natural substances for prevention of skin photoaging: Screening systems in the development of sunscreen and rejuvenation cosmetics. Rejuv. Res. 21(2): 91-101
View at Publisher | View at Google Scholar - Martinez, R. M. Pinho-Ribeiro, F. A. Steffen, V. S. Caviglione, C. V. Fattori, V. Bussmann, A. J. C. (2017). Trans-Chalcone, a flavonoid precursor, inhibits UV-induced skin inflammation and oxidative stress in mice by targeting NADPH oxidase and cytokine production. Photochem. Photobiol. Sci. 16(7):1162-1173
View at Publisher | View at Google Scholar - Martinez, R. M. Pinho-Ribeiro, F. A. Vale, D. L. Steffen, V. S. Vicentini, F.T. Vignoli, J. A. (2017). Trans-chalcone added in topical formulation inhibits skin inflammation and oxidative stress in a model of ultraviolet B radiation skin damage in hairless mice. J. Photochem. Photobiol. B: Biology. 171:139-146.
View at Publisher | View at Google Scholar - Tom, D. K., Immeyer, J. Wolber, R. Kolbe, L. (2005). Anti-inflammatory properties of licochalcone a from Glycyrrhiza inflata on various human skin cells. J. Am. Acad. Dermatol. 52(3): P1055.
View at Publisher | View at Google Scholar - Kolbe, L. Immeyer, J. Batzer, J. Wensorra, U. Dieck, K. T. Mundt, C. (2006). Antiinflammatory efficac of licochalcone A: Correlation of clinical potency and in vitro effects. Arch. Dermatol. Res. 298(1):23-30.
View at Publisher | View at Google Scholar - Kühnl, J. Roggenkamp, D. Gehrke, S. A. Stäb, F. Wenck, H. Kolbe, L. (2015): Licochalcone A activates Nrf2 in vitro and contributes to licorice extract-induced lowered cutaneous oxidative stress in vivo. Exper. Dermatol. 24(1):42-47.
View at Publisher | View at Google Scholar - Immeyer, J. Batzer, J.Wolber, R. Kolbe, L.(2005). Anti-irritative efficacy of licochalcone A-containing formulations on razor- and UV-induced skin irritation. J. Am. Acad. Dermatol.52(3): P1007.
View at Publisher | View at Google Scholar - Petrova, A. Davids, L. M.; Rautenbach, F. Marnewick, J. L. (2011). Photoprotection by honeybush extracts, hesperidin and mangiferin against UVB-induced skin damage in SKH-1 mice. J. Photochem. Photobiol. B: Biology.103(2).126-139.
View at Publisher | View at Google Scholar - Martinez, R. M. Pinho-Ribeiro, F. A.Steffen, V. S.; Caviglione, C. V. Pala, D. Baracat, M. M.(2016). Topical formulation containing hesperidin methyl chalcone inhibits skin oxidative stress and inflammation induced by ultraviolet B irradiation. Photoch. Photobiol. Sci.15(4): 554-563.
View at Publisher | View at Google Scholar - Forestier, S. Moire, C.Lang, G.(1988). Preparation of chalcone derivatives as sunscreens. Ger. Offen.
View at Publisher | View at Google Scholar - Fujii, A. Sashita, Y. Mimaki, Y. Matsubara, K. Hara, R. Kitada, Y. Nakajima,T. Oosato, Y. Oohata, S.(1996). Myata, Y. 2,4,6-Trihydroxychalcone as a UV absorbing agent and cosmetics containing it. Jpn. KokaiTokkyoKoho.
View at Publisher | View at Google Scholar - Imokawa, G. Takaishi, N. Tejima, T. Nakamura, K. (1995). Hattori, M. Masuda, S. Chalcone derivatives and an ultraviolet absorber containing them. Ger. Offen.
View at Publisher | View at Google Scholar - Okuya, F. Nishio, H. Kuwamura, S. Deguchi, Y. (1993). Chalcone derivatives and copolymers thereof as UV absorbers. Jpn. KokaiTokkyoKoho.
View at Publisher | View at Google Scholar - Lahorkar, P.G.R. Vaidya, A.A. Chavan, M.V. Gadgil, V.R. (2014). A Photoprotective personal care composition. PCT Int. Appl.
View at Publisher | View at Google Scholar - Quintana, S. Svarc, F. Sagrera, G. Dicelio, L. (2018). Absorption and photo-stability of substituted dibenzoylmethanes and chalcones as UVA filters. Cosmetics.
View at Publisher | View at Google Scholar - Sagrera, G. Bertucci, A. Vazquez, A. Seoane, G. (2011). Synthesis and antifungal activities of natural and synthetic biflavonoids. Bioorg. Med. Chem.19:3060- 3073.
View at Publisher | View at Google Scholar - Sagrera, G. Seoane, G. (2010). Total synthesis of 3’3’-binaringenin and related biflavonoids. Synthesis. 16:2776–2786.
View at Publisher | View at Google Scholar - Karlsson, I. Hillerstrom, L. Stenfeldt, A.L. Martensson, J. Borje, A. (2009). Photodegradation of dibenzoylmethanes: Potential cause of photocontact allergy to sunscreens. Chem. Res. Toxicol. 22, 1881-1892.
View at Publisher | View at Google Scholar - Sayre, R.M. Dowdy, J.C. Gerwig, A.J. Shields, W.J. Lloyd, R.V. (2005). Unexpected photolysis of the sunscreen octinoxate in the presence of the sunscreen avobenzone. Photochem. Photobiol. 81:452–456.
View at Publisher | View at Google Scholar - Pfeifer, G.P. Besaratinia, A. (2021). UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem. Photobiol. Sci.11:90–97.
View at Publisher | View at Google Scholar - Shaath, N.A. (2006). Ultraviolet filters. Photochem. Photobiol. Sci. 9:464–469. Gonzenbach, H. Photostability of Cosmetic sunscreens. In Pharmaceutical Photostability and Stabilization Technology; Piechocki, J. Thoms, K. Eds. Informa Healthcare: New York, NY, USA, pp. 379–396.
View at Publisher | View at Google Scholar

 Clinic
Clinic
