Research Article | DOI: https://doi.org/10.31579/2835-9216/026
Role Toll-like Receptor 4 gene Underlying Hypoxia-Inducible transcription Factor 1α Gene Expression in Cervical Cancer of Women
*Corresponding Author: Mustafa Adnan Nama, University of Maysan, Amarah, Iraq.
Citation: Mustafa Adnan Nama, Qayssar Ali Kraidi, (2024), Role Toll-like Receptor 4 gene Underlying Hypoxia-Inducible transcription Factor 1α Gene Expression in Cervical Cancer of Women, Carcinogenesis and Chemotherapy, 3(3); DOI:10.31579/2835-9216/026
Copyright: © 2024, Mustafa Adnan Nama. This is an open-access artic le distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 07 May 2024 | Accepted: 16 May 2024 | Published: 27 May 2024
Keywords: TLR4; HPV; HIF-1α; and cervical cancer
Abstract
Molecular methods are being used in cervical cancer screening to identify high-risk human papillomavirus. Annually, 490,000 new infections are diagnosed with papillomavirus, and about 298,000 death cases due to cervical cancer worldwide. Pap and biopsy samples from 65 samples from cervixes were collected from women with histological changes from Alsider Teaching Hospital, Maysan Hospital for Children, South of Iraq. The viruses were detected by RT-PCR in 69.2 % of the all cases. Extracted RNA samples were used to analyze the gene expression of Toll-like receptor 4 and hypoxia-inducible transcription factor 1α from biopsy samples. It was found that the number of positive samples for viruse genotype 16 was in 30 samples, which represents 66.6 %, while the genotype 18 was detected in 17 samples, which represents 37.7%, while 9 samples gave positive results for genotype 31 and represented about 20 %, relying on E6 or E7 specific primers in cDNA template by real-time PCR ,and the values of Toll-like receptor 4 in negative samples were normalized to the housekeeping gene (β actin) to 1 and its values in positive samples were compared to that 1 which was more roughly in 1-fold, and the hypoxia-inducible transcription factor 1α gene expression in positive samples was more in roughly 48-fold compared to its value in the housekeeping gene (β actin) to 1 (negative control). The study concluded that toll-like receptor 4 and hypoxia-inducible transcription factor 1α are overexpressed in cervical cancer, and both synergistically promote the development of cervical cancer.
Introduction
Cervical cancer is the fourth most frequent cancer among women worldwide, following breast cancer, colorectal cancer, and lung cancer [(1]. One of the most prevalent viruses in the world is the human papillomavirus. At least 14 different genotypes have been linked to cancer. The infection starts shortly after the start of sexual activity and is primarily spread through sexual contact. The most lethal genotypes for this virus are types 16 and 18, where female cervical cancer rates of infection are 70% [2]. Every one HPVs are double-stranded DNA viruses without an envelope,their circular genomes are roughly 8 kilobases in size.More than 100 genotypes have been identified, such as HPV16, 18, 31, 32, 45, and 52 can affect the genital tract. relying of the transformation ability of HPV can be split into groups; high-risk and low-risk of HPV genotypes [3]. There are more than 41 HPV genotypes that are easily contagious and can result in genital infection [1].
The Toll-like receptors (TLRs) can be split into two classes [(4]. rely on subcellular situation TLR1, 2, 4, 5, and 6 are sitting on the cell surface and mainly recognize the lipid composition of the surface of the pathogen, whereas the TLR3, 7, 8, and 9 localize to the cytoplasm and fundamentally distinguish the nucleic acid of pathogens, TLR4 is a critical receptor on which infectious and non-infectious stimuli converge to cause a pro-inflammatory response [5]. TLR4-mediated inflammation, induced by exogenous or endogenous ligands, is also engaged in a variety of acute and chronic illnesses, playing an important function as an inflammatory response amplifier. TLR4 has recently been shown to be important in the recognition of chemical substances produced by damaged tissues and necrotic cells. These molecules, known as damage-associated molecular pattern molecules (DAMPs), activate a robust proinflammatory response when they bind with TLR4[6].
Hypoxia, a type of cellular stress that has been identified in many solid tumors, is crucial for the development of tumors as well as for their growth, invasion, metastasis, and resistance to currently available treatments. The transcription factor hypoxia-inducible factor-1 (HIF-1), which is linked to the unregulated growth of cervical cancer cells [7]. HIF-1 is regarded as a trigger for hypoxia since it plays a significant role in the formation of many cancers [8]. The use of (RT-PCR) to assess the mRNA levels of the target genes needs to use the housekeeping genes such as β-actin [9]. It is presumed that the expression of these genes keeps constant normalizing the variations in signal estimation and processing [10].
The study's objectives included detecting the presence of HPV DNA in women's cervixes, what genotyping of HPV with high risk, Additionally, the vaccination is not effective against the 30% of cervical cancers brought on by other high-risk HPV varieties, thus new cases of the illness will continue to occur and demand medical attention, and the correlation between toll-like receptor 4 (TLR 4) expression and hypoxia-inducible transcription factor 1α (HIF-1α) expression in cervical cancer tissues and their critical roles in initiating the tumors, progression, metastasis, and tumor resistance to the available therapies. It is crucial to keep up study efforts to understand the fundamental biology of these viruses so that novel antiviral treatments might be created.
2. Materials and Methods
2.1. Samples collection.
Pap smear and biopsy samples were collected from 65 women from Alsider Teaching Hospital, Masan Hospital for Children, South of Iraq, for Women and Children, from September (2022) to March (2023). The Biopsy samples were collected from women suffering from different clinical cervical, and uterine abnormalities, and carcinoma. Positive or negative samples were categorized based on the presence or absence of HPV DNA in the collected samples.
2.2. Molecular detection of HPV genotypes by Real-Time PCR.
The detection of high-risk HPV genotypes was performed by real-time PCR using RealMOD™ Green SF 2X qPCR mix kit from iNtRON Biotechnology, according to the manufacturer's instructions. The total volume of reaction 20 µl was prepared by mixing 2µl of cDNA template with 10 µl of SYBR Green Master Mix, 1 µl of each forward and reverse primers of HPV16 E7, HPV18 E7, and HPV31 E6, and 6 µl of Free DEPEC-D.W. The Real-Time PCR conditions were set as follows: the reaction was submitted to the first step of denaturation at 95˚C for 10 min followed by 40 cycles of 15 sec of denaturation at 95 ˚C, the annealing step was set at 60 ˚C for 30 sec, an extension step at 72 ˚C for 30 sec with a final extension conducted at 72˚C for 5 min. The reaction was subjected to the melting curve analysis to ensure primer specificity. The primers used for qPCR were as follows: HPV16 E7; F- (5' GAACCGGACAGAGCCCATTA 3'), R-(5'ACACTTGCAACAAAAGGTTACA3′), forHPV18E7; F-(5'CAACGTCACA CAATGTTGTGA3'), R-(5'TCAATTCTGGCTTCACACTTAC3'), and, forHPV31E6; F-(5'GGTCAGTTAACAGAA ACAG AGG3′), R-(5'TGGTGTGTCGTCCC TATATACT 3′)
2.3 Molecular detection of TLR4, HIF-1α, and β-actin gene by Real-Time PCR.
Total RNA from the cervical cancer samples was extracted using an RNA extraction kit (Promega, USA) according to the manufacturer’s instructions. The extracted total RNA was quantified by absorbance at 280 nm using a NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), The mRNA in the total RNA was reverse transcribed to complementary DNA using a cDNA synthesis Kit (Promega, USA). The qPCR was performed in the I Cycler iQ5 (Bio-Rad) using SYBR RT per mix (England). The total volume of reaction 20 µl was prepared by adding 4µl of cDNA template with 10 µl of SYBR Green Master Mix, 1 µl of each forward and reverse primers of TLR4, HIF-1α, and β-actin, and 4 µl of Free DEPEC-D.W. The Real-Time PCR conditions for each of TLR4, HIF-1α, and β-actin were set as follows: the reaction was submitted to the first step of denaturation at 95˚C for 10 min followed by 45 cycles of 15 sec at 95˚C of denaturation, annealing step was set at 59 ˚C for 30 sec, an extension step at 72 ˚C for 30 sec with a final extension conducted at 72˚C for 5 min. The reaction was subjected to the melting curve analysis to ensure primer specificity. All real-time PCRs were performed in triplicate. The mRNA expression levels, which were normalized against β-actin, were calculated and expressed as ΔΔCT. The primers used for qPCR were as follows: β-actin; F- 5ʹ-GATTACTGCTCTGGCTCCTAGC-3ʹandR-5ʹ-GACTCATCGTACTCCTGCTTGC-3ʹ, forTLR4:F5′CCCTGAGGCATTTAGGCAGCTA-3′, and, R-5′-AGGTAGAGAGGTGGCTTAGGCT-3′, for, HIF1α: F-5ʹ-TATGAGCCAGAAGAACTTTTAGGC-3ʹ, and, R-5ʹ-CCCAGG TCCTCGCTT ATGATCT-3ʹ.
3. The Results
3.1. Detection of genotypes.
The detection of the high-risk HPV16, HPV18, and HPV31 for 45 samples from total 65 samples relying on E6 or E7 specific primers in cDNA template by real-time PCR, it was found that the number of positive samples for genotype 16 is 30 samples, which represents 66.6 %, while the genotype 18 was detected in 17 samples, which represents 37.7%, while 9 samples gave positive results for HPV31 and represented about 20 %.
3.2. Relative expression of target genes by qPCR.
3.2.1. Relative gene expression of TLR4.
To measure the gene expression of TLR4 which plays an important role in the recognition of endogenous and exogenous ligands. The gene expression of the TLR4 through the reaction consisting of mixing SYBR green dye and specialized primers with cDNA. The data showed that the gene expression of TLR4 was high in positive samples of more than 2 CTs before the negative sample signal after subtracting the signal of the housekeeping gene (β-actin). The ΔΔ CTs analysis was adopted here by subtracting the housekeeping gene. Figure (1). Moreover, to exactly detect the fold change, the values of TLR4 in negative samples were normalized to 1 and its values in positive samples were compared to that 1 which was more roughly in 1-fold.
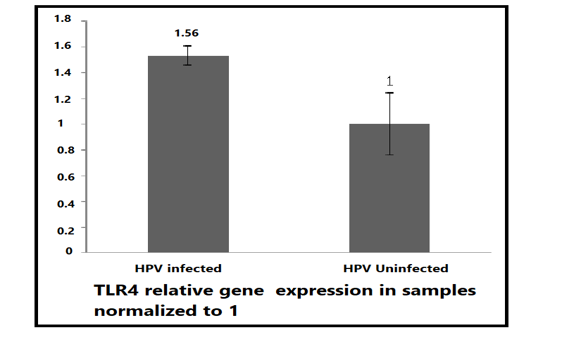
Figure 1: TLR4 relative gene expression in positive and negative samples. Total RNA was extracted, reverse transcribed and the synthesized DNA was used as a template for qPCR relative expression assay using SYBR green master mix. Data were analyzed by ΔΔ CTs and normalized to (β actin) house-keeping gene which was equalized to 1. (t-test P= 0.0031 significant).
3.2.1 Relative gene expression of HIF-1α.
To assess the gene expression and role of HIF-1α in the uncontrolled growth of cancer cells in HPV infection, RNA samples that were reverse transcribed into a cDNA template were mixed with primers amplifying HIF-1α to measure its gene expression using qPCR SYBR green. Obviously, the expression level of HIF-1α was significantly higher in positively diagnosed samples compared to the negative samples. The fluorescence signal of HIF-1α for the negative sample delayed more than 14 CTs behind the positive sample signal after subtracting the signal of the β-actin. The data then were analyzed by normalizing the negative control samples to 1 then calculating the difference of folds in expression, its expression in positive samples was more in approximately 48-fold compared to its value in the negative control Figure (2).
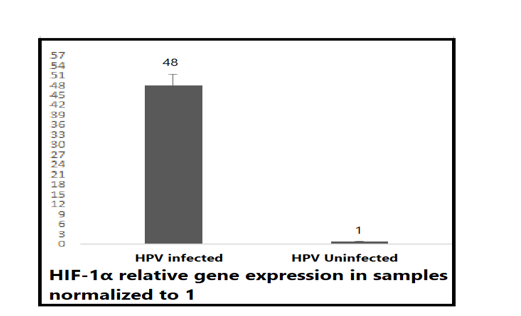
Figure 2: HIF-1α relative gene expression in positive and negative samples. Total RNA was extracted, reverse transcribed and the synthesized DNA was used as a template for qPCR relative expression assay using SYBR green master mix, the gene expression of HIF-1α was increased around 48-fold in positive samples compared to that 1. Data were analyzed by ΔΔ CTs and normalized to (β actin) house-keeping gene which was equalized to 1. (t-test P= 0.0038significant).
4. Discussion
Molecular detection by real-time PCR in the present showed the HPV16, HPV18, and HPV31 genotypes. Genotype 16 was detected in 66.6 %, followed by genotype 18 in 37.7%, while 9 samples gave positive results for HPV31 and represented 20 %. In fact, these results indicated an increase in HPV prevalence in women, especially high-risk genotypes 16 and 18. The study also found mixed infection with more than one genotype within the same sample of the 18 (41%) samples, were infected with one genotype, while 27 (59%) were found to be infected with more than one genotype. These results were consistent with [11]. where they demonstrated the presence of multiple infections reached 6 genotypes. Consequently, finding two or more HPV genotypes in a single infected sample is not rare [5].
According to recent findings, viral infection causes the gene expression of TLR4 to be highly expressed in positive samples, the high level of TLR4 gene expression has an obvious association with tumors resulting from the transformation of squamous cells of the cervix into immortal cells by the carcinogenic proteins of the HPV [12]. when compared with negative samples of cervix tissue, which compatible with studies by [13]. it’s found that TLRs, particularly TLR4, TLR5, and TLR9, are very associated with HPV infection, and cervical cancer cells may grow faster if TLR4 is activated. Studies investigated, the connection between TLR4 and cervical cancer cells in vitro [13]. and discovered that TLR4 supported HPV-related cervical cancer cells in proliferating and resisting apoptosis [14]. this shows that the high level of TLR4 gene expression in positive cases was connected with HPV infection. where the level of TLR4 gene expression was lower in negative than the positive samples, Additionally, when the TLR4/MyD88/NF-B pathway is activated, proinflammatory cytokines are abundantly produced in HPV-related cervical cancer cells [15]. The S100A8/S100A9 as an endogenous TLR4 activator, may encourage tumor growth and metastasis [16]. The binding of receptors TLRs to HPV infection may result from E6 regulating several transcriptional pathways [(17]. which affects the immune system's capacity to recognize pathogens, thus enabling HPV to escape from immune surveillance [18]. This suggests that various HR-HPV varieties have distinct regulatory mechanisms for controlling TLRs [19].
Many tumors have low O2 concentrations [20]. Hypoxia, known as the O2 concentration of tissues less than 1.5%, which plays a major role in the progression and development of the tumors [17]. Clinically, hypoxia can increase resistance to radiotherapy and chemotherapy and is considered a negative predictive sign of many cancers, including the positive tumors of the HPV. The availability of O2 is known to influence tumor cell biology. Cervical cancers often show very low O2 content, with a heterogeneous distribution of more and less oxygenated regions and an average concentration of O2 at 1.2% [21]. Hypoxia microenvironments are present in the solid tumor tissue [22]. excessive activation of the HIF-1α in microenvironments has a close correlation with the occurrence and development of cervical cancer and uncontrolled growth of cervical cancer cells, and these microenvironments show a high level of the hypoxia-inducible factor-1α [21].
The results of this study showed a high level of HIF-1α expression in positive cases of HPV infection compared to the negative samples which show a low level of expression. Through advanced results, we note that there is a clear relationship between TLR4 and HIF-1α gene expression in positive cases of cervical cancer [23]. These results were compatible with the results of the researcher, as they indicated the presence of the close association of HIF-1α with TLR4 in cervical cancer caused by HPV infection, and detected changes in the RNA content of HIF-1α in each group to explore their correlation. The RNA content of HIF-1α was similar to TLR4, and a suggestion from these results was that the expression HIF-1α was positively associated with the expression of TLR4 in the cervix [24].
Conclusion
In summary, the study concluded that Toll-like receptor 4 and hypoxia-inducible transcription factor 1α are overexpressed in cervical cancer, and both synergistically promote the development of cervical cancer. It is critical to continue research efforts to understand the underlying biology of these viruses in order to develop new antiviral medications.
Declarations
Declarations Conflict of interest
According to the authors, the research was carried out without any commercial or financial ties that might be viewed as potential conflicts of interest
Acknowledgements
We appreciate and thank the physicians and nursing staff at Alsider Teaching Hospital and Maysan Children's Hospital, southern Iraq, who assisted in managing recruitment and follow-up for women.
Author contributions
Nama Mustafa Adnan conducted the experiments, analyzed experimental data, helped conduct the RT-PCR, and designed the figures. Pap and biopsy samples collected from patients via the physicians and nursing staff at Alsider Teaching Hospital and Maysan Children's Hospital, southern Iraq, Nama Mustafa Adnan and Qayssar Ali Kraidi were responsible for manuscript revision and total RNA was extracted, reverse transcribed and the synthesized DNA as a template for qPCR.
Funding
This study was supported by the Strategic Priority Research Program of the IRAQ Academy of Sciences, college Sciences in maysan university
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Committee on Human Research Publications and Ethics of Alsider Teaching Hospital, Maysan Hospital for Children, South of Iraq, and Committee on Human Research Publications, and Ethics of maysan University, South of Iraq.
References
- Wang X, Song Y, Wei X, Wang G, Sun R, Wang M, Zhao L. (2022). Prevalence and distribution of human papillomavirus genotypes among women attending gynecology clinics in northern Henan Province of China. Virol J 19(1):6.
View at Publisher | View at Google Scholar - Kombe Kombe AJ, Li B, Zahid A, Mengist HM, Bounda G-A, Zhou Y, Jin T. (2021). Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front Public Health 8.
View at Publisher | View at Google Scholar - Yu H, Yi J, Dou Y-L, Chen Y, Kong L-J, Wu J. (2021). Prevalence and Genotype Distribution of Human Papillomavirus Among Healthy Females in Beijing, China, 2016–2019. Infect Drug Resist 14:4173-4182.
View at Publisher | View at Google Scholar - El-Zayat SR, Sibaii H, Mannaa FA. (2019). Toll-like receptors activation, signaling, and targeting: an overview. Bull Natl Res Centre 43(1):187.
View at Publisher | View at Google Scholar - Yang X, Cheng Y, Li C. (2017). The role of TLRs in cervical cancer with HPV infection: a review. Signal Transduct Target Therapy 2(1):17055.
View at Publisher | View at Google Scholar - Chen X, Zhang Y, Fu Y. (2022). The critical role of Toll-like receptor-mediated signaling in cancer immunotherapy. Med Drug Discovery, 14:100122.
View at Publisher | View at Google Scholar - Xiong J, Nie M, Fu C, Chai X, Zhang Y, etal. (2022). Hypoxia Enhances HIF1α Transcription Activity by Upregulating KDM4A and Mediating H3K9me3, Thus Inducing Ferroptosis Resistance in Cervical Cancer Cells. Stem Cells International, 1608806.
View at Publisher | View at Google Scholar - Peng X, Gao H, Xu R, Wang H, Mei J, Liu C. (2020). The interplay between HIF-1α and noncoding RNAs in cancer. J Experimental Clin Cancer Res 39(1):27.
View at Publisher | View at Google Scholar - Nowak M, Aslan S, Kowalewski MP. (2020). Determination of novel reference genes for improving gene expression data normalization in selected canine reproductive tissues – a multistudy analysis. BMC Vet Res 16(1):440.
View at Publisher | View at Google Scholar - Mu J, Wang Y, Wang M, Zhang D, Liu M. (2023). Identification of reliable reference genes for gene expression studies in mouse models under microplastics stress. Ecotoxicol Environ Saf 252:114569.
View at Publisher | View at Google Scholar - Nikolic N, Basica B, Mandic A, Surla N, Gusman V, etal. (2023). E6/E7 mRNA Expression of the Most Prevalent High-Risk HPV Genotypes in Cervical Samples from Serbian Women. Diagnostics (Basel Switzerland) 13(5):917.
View at Publisher | View at Google Scholar - Feng L, Lintula S, Ho TH, Anastasina M, Paju A, et al. (2012). Technique for strand-specific gene-expression analysis and monitoring of primer-independent cDNA synthesis in reverse transcription. Biotechniques 52(4):263-270.
View at Publisher | View at Google Scholar - Jiang N, Xie F, Chen L, Chen F, Sui L (2020) The effect of TLR4 on the growth and local inflammatory microenvironment of HPV-related cervical cancer in vivo. Infect Agents Cancer 15(1):12.
View at Publisher | View at Google Scholar - Morale MG, Tamura RE, Cintra R, Araújo NM, Villa LL. (2022). TLR4 and SARM1 modulate survival and chemoresistance in an HPV-positive cervical cancer cell line. Sci Rep 12(1):6714.
View at Publisher | View at Google Scholar - Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, Li Y. (2021). Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Therapy 6(1):263.
View at Publisher | View at Google Scholar - Deguchi A, Watanabe-Takahashi M, Mishima T, Omori T, Ohto U, etal. (2023). Novel multivalent S100A8 inhibitory peptides attenuate tumor progression and metastasis by inhibiting the TLR4-dependent pathway. Cancer Gene Ther.
View at Publisher | View at Google Scholar - Cruz-Gregorio A, Aranda-Rivera AK. (2021). Redox-sensitive signalling pathways regulated by human papillomavirus in HPV-related cancers. Rev Med Virol, 31(6) :2230.
View at Publisher | View at Google Scholar - Haręża DA, Wilczyński JR, Paradowska E. (2022). Human Papillomaviruses as Infectious Agents in Gynecological Cancers. Oncogenic Properties of Viral Proteins. In International Journal of Molecular Sciences.
View at Publisher | View at Google Scholar - Steinbach A, Riemer AB. (2018). Immune evasion mechanisms of human papillomavirus: An update. Int J Cancer ,142(2):224-229.
View at Publisher | View at Google Scholar - Vaupel P, Flood AB, Swartz HM. (2021). Oxygenation Status of Malignant Tumors vs. Appl Magn Reson 52(10):1451–1479. Normal Tissues: Critical Evaluation and Updated Data Source Based on Direct Measurements with pO2 Microsensors.
View at Publisher | View at Google Scholar - Avila JP, Carvalho BM, Coimbra EC. (2023). A Comprehensive View of the Cancer-Immunity Cycle (CIC) in HPV-Mediated Cervical Cancer and Prospects for Emerging Therapeutic Opportunities. Cancers, 2072-6694.
View at Publisher | View at Google Scholar - Harris B, Saleem S, Cook N, Searle E. (2022). Targeting hypoxia in solid and haematological malignancies. J Experimental Clin Cancer Res 41(1):318.
View at Publisher | View at Google Scholar - Zhang T, Yang J, Sun Y, Song J, Gao D, et al. (2023) Interleukin-6 and Hypoxia Synergistically Promote EMT-Mediated Invasion in Epithelial Ovarian Cancer via the IL-6/STAT3/HIF-1α Feedback Loop. Analytical Cellular Pathology, 8334881.
View at Publisher | View at Google Scholar - Priego-Hernández, V. D, Arizmendi-Izazaga, A, Soto-Flores, D. G, Santiago-Ramón,N, Feria-Valadez, M. D, et al.(2023). Expression of HIF-1α and Genes Involved in Glucose Metabolism Is Increased in Cervical Cancer and HPV-16-Positive Cell Lines.In Pathogens.
View at Publisher | View at Google Scholar

 Clinic
Clinic
