Review Article | DOI: https://doi.org/10.31579/2835-9232/066
Relationship of NK cells and Killer Immunoglobulin-like Receptors with Leukemia’s
1Director, Department of Transplant Biology, Shrimann Superspeciality Hospital, Jalandhar -144012, Punjab, India*
*Corresponding Author: Suraksha Agrawal, 1Director, Department of Transplant Biology, Shrimann Superspeciality Hospital, Jalandhar -144012, Punjab, India*
Citation: Suraksha Agrawal, Dinesh Chandra, (2024), Relationship of NK cells and Killer Immunoglobulin-like Receptors with Leukemia’s, International Journal of Clinical Epidemiology, 3(4); DOI:10.31579/2835-9232/066
Copyright: © 2024, Suraksha Agrawal1. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 14 May 2024 | Accepted: 10 June 2024 | Published: 08 July 2024
Keywords: natural killer cells; killer immunoglobulin receptors (kirs); human leukocyte antigens (hla), leukemia, acute lymphocytic leukemia (all); acute myeloid leukemia (aml); chronic lymphocytic leukemia(cll); and chronic myelogenous leukemia (cml)
Abstract
Natural Killer (NK) cells are immune cell belonging to innate immunity that are very effective in killing abnormal cells such as cancer cells and cells infected with viruses. NK cells are found in a variety of tissues and have different characteristics. NK cells have both activating and inhibitory receptors that allow them to recognize abnormal cells and regulate their killing activity. In clinical trials, NK cells have been used to suppress the growth of tumors, but the results have only been positive in blood cancers and not in solid tumors. The type of tumor and its environment can influence the activity of NK cells. In this review, we explore the role of different NK cell receptors and how they impact different types of blood cancers, as well as their role in immunotherapies.
Introduction
The body is protected by our immune cells like lymphocytes (T, B and NK cells, neutrophils, monocytes and macrophages and their proteins. When these cells are not functioning normally, they cause different diseases including cancers. Interestingly cancer cells have an escape mechanism as a result of this there may be immune tolerance because of which cytotoxic innate and adaptive immune cells may not attack the malignant cells. NK cells have inhibitory and stimulatory receptors that help in immune surveillance. Some of the cancer cells lack MHC-I leading to programmed cell death. Interestingly NK cells have an anti-tumour effect [1].
In this review, we will discuss the role of NK cells and their receptors in blood cell malignancies
NK cells
NK cells, making up 10% of circulating lymphocytes, are large, granular, bone marrow-derived lymphocytes with a CD56+ CD3 phenotype. They play a crucial role in the innate immune response and are involved in infectious diseases, pregnancy, autoimmunity, cancer, and bone marrow transplantation [2, 3, 4]. The Killer Immunoglobulin-like Receptor (KIR) family, including Natural Killer Group 2A (NKG2A) and Leukocyte Immunoglobulin-like Receptor Subfamily B Member 1 (LILRB1), plays a crucial role in NK cell education, enabling self-inhibited NK cells to become potent killers [5, 6]. NK cells are activated by various receptors resulting in the natural cytotoxicity [7], including the natural cytotoxicity receptors (NCRs) NKp30 and NKp46, NKG2D, 2B4, and DNAM-1, following binding to ligands up-regulated during stress and infection. Degranulation requires at least two receptors to be stimulated simultaneously, unlike the FcγRIIIA receptor (CD16a), which can trigger degranulation upon ligation to the Fc portion of an antibody [8] that may lead to cell lysis.
NK cells are activated by various receptors, including natural cytotoxicity receptors (NCRs) NKp30 and NKp46, NKG2D, 2B4, and DNAM-1, following binding to ligands up-regulated during stress and infection. Antibody-dependent cellular cytotoxicity (ADCC) involves NK cells engaging the LFA-1 receptor to direct granular release towards target cells, triggering efficient target lysis [8,9,10]. Target cells can undergo apoptosis when exposed to TRAIL and FasL on NK cells, and can control DC-mediated cross-priming by TRAIL/DR5 [11].
NK cells detect MHC class I on host cell surfaces through interaction with KIRs. Target cells have receptors like missing-self, induced-self, and altered-self (12,13). The activating receptors may identify pathogen-encoded ligands or the peptide repertoire in MHC class I molecules, inhibitory receptors block the activation of NK cells. In severe infections or malignancies, HLA antigen expression may be down-regulated, resulting in cells lacking HLA-specific ligands. NK cells express inhibitory receptors for self-MHC class I ligands, preventing cell lysis (14, 15). They recognize and attack cells without self-antigens, known as the 'missing self-hypothesis' (Figure 1).
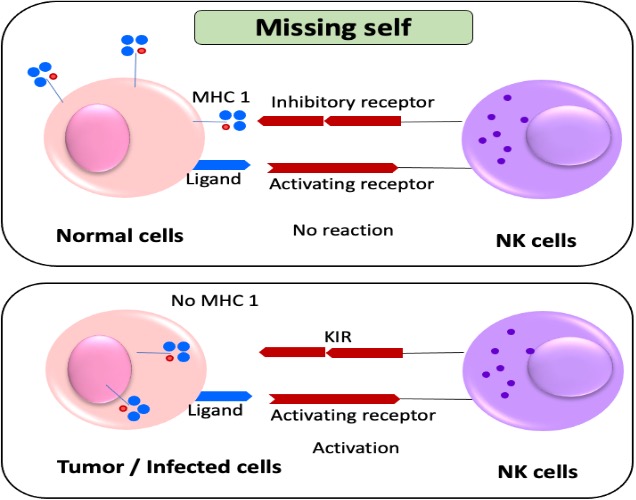
NK cells recognize and eliminate tumors or infected cells lacking MHC-I expression, using highly polymorphic HLA-A, -B, and -C. CD94/NKG2A and are involved in self-tolerance [16]. The genetic analysis of human populations showed CD94:NKG2A mediates NK cell education, response, and recognition. The role of both non-classical and classical antigens requires ligand specificity of inhibitory and activating receptors (Table 1)
Type | Activating receptor | Activating receptor ligand specificity | Inhibitory receptors | Inhibitory receptor ligand specificity |
Killer immunoglobulin receptors | KIR2DS1 | Group 2 HLA-C Asn77 Lys80 | KIR2DL1 (CD158a) | Group 2 HLA-C Asn77Lys80 |
| KIR2DS2 | Group 1 HLA-C Ser 77 Asn80 | KIR2DL2 (CD158b) | Group 1 HLA-C Ser77Asn80 |
| KIR2DL4 | HLA-G | KIR2DL3 (CD158b) | Group 1 HLA-C Ser77Asn80 |
| KIR2DS4 | Unknown | KIR3DL1 | HLA-Bw4 |
| KIR2DS5 | Unknown | KIR3DL2 | HLA-A3, -A11 |
| KIR3DS1 | Unknown | KIR3DL7 | Unknown |
C-type lectin receptors | CD94/ NKG2C | HLA-E | CD94/ NKG2A/ B | HLA-E |
| CD94/ NKG2E/H | Unknown | CIRU | CIRU |
| NKG2D | MIC-A, MIC-B, ULBP-1, 2 & 3 | CIRU | CIRU |
Natural cytotoxicity | NKp46, NKp44, | Unknown | Unknown | CIRU |
Receptors | NKp30 |
|
|
|
Table 1: Human activating and inhibitory NK cell receptors (NKR) and their corresponding ligands
Killer cell immunoglobulin-like receptors (KIR) NK and T cells have a family of 17 genes on the leukocyte receptor complex called KIRs on their surface. They participate in both immune responses, making them vital parts of the immune system. Because KIR genes are exclusive to HLA class I allotypes, variations in these genes can impact resistance to and susceptibility to hematological malignancies. Due to regular reorganization and reciprocal and non-reciprocal crossing-over [17], KIR haplotypes are extremely diverse, with all people belonging to four "framework" genes (KIR3DL3, KIR3DP1, KIR2DL4, and KIR3DL2) [18]. KIR haplotypes are categorized into A and B. A haplotype haveless gene content and mostly encoding inhibitory receptors. The B haplotype is longer and has more activating receptors [19] (Supplementary Figure-1).
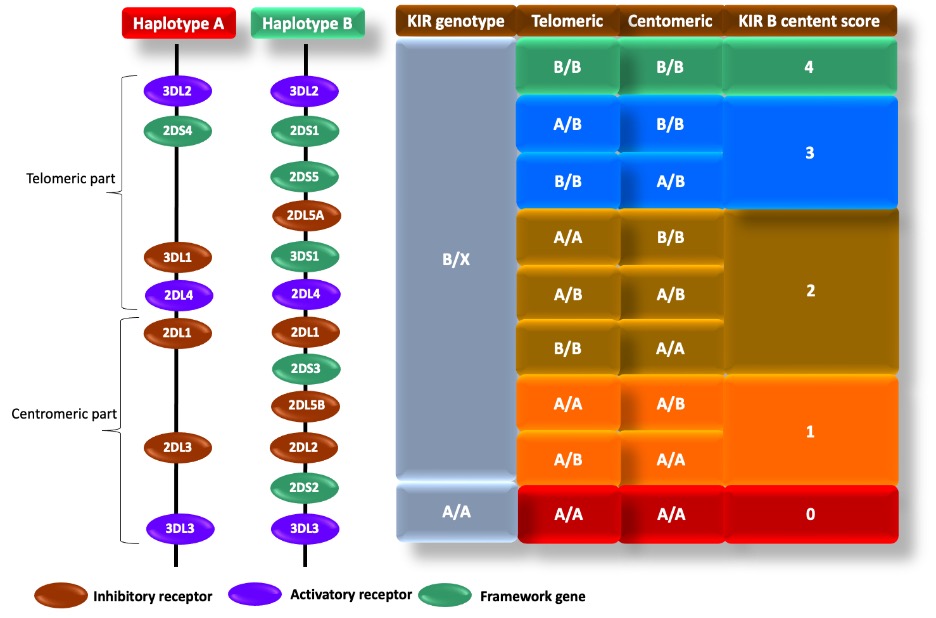
Supplementary Figure-1: The KIR framework genes, activating and inhibitory receptors
Inhibitory KIRs have long cytoplasmic domains with ITIMs that recruit protein tyrosine phosphatases. Short cytoplasmic domains associate with DAP12, a transmembrane signaling adaptor protein, which activates DAP12-dependently through Syk/ZAP-70 tyrosine kinases, a process consistent with antigen receptor signaling [20]. The only exception to the short- or long-tailed rule is KIR2DL4, a unique long-tailed KIR that activates cytokine production instead of cytotoxicity. It is associated with an ITAM-containing FceRI-c adaptor instead of DAP12 (21). KIR molecules recognize specific MHC-I allotypes, with inhibitory binding having higher affinity. Activating KIRs are more responsive to allogeneic MHC-I, potentially triggering anti-tumor responses in NK cells after hematopoietic transplantation [22]. Different KIR receptors, ligand groups, and members are shown in Table 2.
KIR receptor | KIR Ligand Group | Ligand group member |
Activating | ||
2DS1 | HLA-C group 2 | C*02, C*04, C*05, C*06 |
2DS2 | HLA-C group 1 | C*01, C*03, C*07, C*08 |
2DS3 | Unknown |
|
2DS4 | HLA-G? | C*04 |
3DS1 | HLA-B Bw4 | B*08, B*13, B*27, B*44, B*51, B*52, B*53, B*57, B*58 |
2DL4 | HLA-G? |
|
Inhibitory | ||
2DL1 | HLA-C group 2 | C*02, C*04, C*05, C*06 |
2DL2 | HLA-C group 1 | C*01, C*03, C*07, C*08 |
2DL3 | ||
2DL4 | HLA-G? |
|
2DL5 | Unknown |
|
3DL1 | HLA-B Bw4 | B*08, B*13, B*27, B*44, B*51, B*52, B*53, B*57, B*58 |
3DL2 | HLA-A | A*03, A*11 |
3DL3 | Unknown |
|
2DS5 | Unknown |
|
Table 2 Inhibitory and activating receptors, their ligands, and ligand group members.
The KIR promoters on NK cells are unequivocally 120-250bp in size and are indisputably regulated by transcriptional factors such as YY1, CRE/ATF, RUNX3, and Sp1. In contrast, the KIR promoters in T cells are at least 60bp in size and exhibit erratic methylation. Activating KIRs cannot directly
activate T cells unless there is a consistent expression of DAP12. CD56, present on a small subset of T cells, undoubtedly displays reduced proliferation and up-regulates P16 and P53. Most KIR+ T cells are CD56+ and have both NK and CD8+ CTL functions (Figure 2).
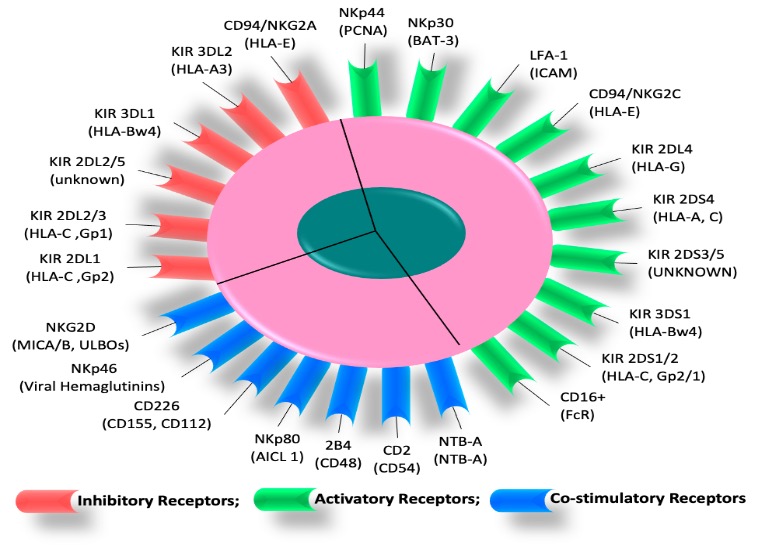
Figure 2: Various activatory, co-stimulatory, and inhibitory receptors in response to leukemia are illustrated on NK cell
Role of NK cell receptors, KIR and other receptors in acute lymphocytic Leukemia
Valenzuela-Vázquez and colleagues conducted a study which identified several factors that contribute to the development of cancer including dysfunctional immune systems, endogenous and exogenous factors, and genetic factors. The study found that high-risk ALL patients [23, 24] have decreased activating and inhibitory receptors, reduced NK cell survival, and lower levels of NKp46+ NK cells. The study also showed that NKG2C identifies HLA-E molecules, which are expressed at reduced levels in ALL patients. Furthermore, the study confirmed the importance of DNAM-1 in identifying and eliminating leukemic cells, as there was a significant decrease in DNAM+ NK cell percentage and overall DNAM expression in ALL patients [25, 26, 27]. The study also found that CD96+ NK cells and expression were significantly reduced in ALL patients, suggesting that CD96 may be crucial for identifying ALL blasts and balancing the DNAM-1-TIGIT-CD96 axis [28]. Finally, the study revealed that NK cells expressing 2B4, NTBA, or SLAM were also reduced in ALL patients. It's a fact: Chinese cases exhibit significantly lower frequencies of the 2DS3 and KIR2DS5 genes. Notably, genotyping of the KIR2DS5 gene uncovered no significant differences between controls and acute lymphoblastic leukemia patients. These results warrant further investigation to determine the precise role of these genes in the onset of acute lymphoblastic leukemia [29].
Augusto's review found conflicting results on KIR genotyping and its role in leukemia susceptibility. Selecting hematopoietic stem cell donors based on KIR alleles may improve immunotherapy efficiency, but sub-typing for KIR2DL1 alleles was not involved in the efficacy [30]. The sub-typing study for KIR2DL1 alleles (KIR2DL1∗001-004) carried out in ALL patients with HLA-C2 unequivocally revealed no significant difference. [31]. It has been established that males with T-cell acute lymphoblastic leukemia (T-ALL) possess distinct polymorphisms at KIR genes (KIR2DL4, KIR2DL1, 3DL2, and 3DL3) when compared to controls. The variants identified in killer lectin-like receptors KLRC1/NKG2A (rs2253849) and KLRC2/NKG2C (rs1141715) in male T-ALL patients are of significant importance as they were detected in almost all patients in one of the studies [32]. LILRB1 is a
highly diverse gene that produces an inhibitory receptor on various hematopoietic cells, including NK cells. Tumor cells may utilize the HLA-G ligand to suppress NK cells by binding to LILRB1. Further, several members of the LILRB family are expressed by neoplastic B cells and T cell ALL. [33, 34] KLDR1 (CD94) interacts with NKG2 proteins, affecting NK cell function. CD94-deficient NK cells can kill target cells, suggesting its role [35]. In female patients with B-ALL show greater activation receptor amplification of activation receptors KLRC2, KLRC4, and NCR3 than males, suggesting a more severe disease with higher inflammation levels, potentially resulting in a more favorable treatment response. [36]
Role of NK cell receptors, KIR and other receptors in AML
Several reports have indicated a significant decrease in the expression of NK activating receptors on circulating NK cells of AML patients, including natural cytotoxic receptors (NCRs), NKG2D, and DNAM-1. [37,38,39,40]. Interestingly, the abnormalities in the phenotype and function of natural killer (NK) cells are partially or entirely restored in patients who achieve remission, indicating that the presence of AML cells causes the abnormalities in NK cells (41). Patients with high expression levels of NKp46 have shown better progression-free survival and overall survival rates than those with low expression levels. The over expression of NKG2A and inhibitory KIRs has been strongly linked to the failure of AML patients to achieve remission [42]. Patients with acute myeloid leukemia (AML) exhibit reduced cell surface expression of natural cytotoxicity receptors (NCRs), resulting in anNCRdull phenotype that adversely affects the function of natural killer (NK) cells and cytokine production. (41) Another mechanism that enables leukemic blast cells to evade immune surveillance is the down regulation of NCR ligands on their cell surface, preventing NCRs from engaging with their respective ligands required to activate NK cell-mediated target lysis [43]. It is well-established that AML patients exhibit better outcomes when NK cells display higher cytotoxicity.
Natural Killer (NK) cells in AML patients exhibit impaired anti-leukemic activity. These mechanisms include down-regulation of activating receptor expression, up-regulation of inhibitory NKG2A expression, down-regulation of NK-activating ligands, secretion of soluble NK-inhibitory factors, and other immunosuppressive mechanisms, the specific molecular mechanisms involved in these alterations are still unclear. An imbalance in the expression of these inhibitory and activating receptors can lead to NK cell dysfunction as illustrated in Figure 3
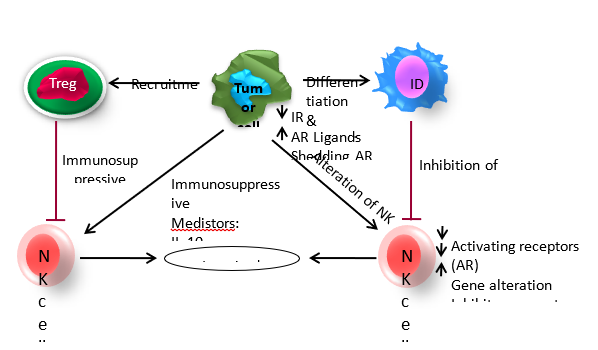
Figure 3: AML cells can escape from NK cells immunosurveillance through various mechanisms. 1). Alteration of NK cells, 2). Immunosuppressive properties of AML cells and 3). Interaction with other immune cells
The programmed cell death ligand-1 (PD-L1) and PD-L2 [44], which show increased levels of AML, causing immune dysfunction (45). The tumor microenvironment also significantly limits the effectiveness of natural killer (NK) cells against AML. The presence of immunosuppressive cells such as Tregs, myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and tolerogenic dendritic cells (DCs) play a critical role in AML progression. There are immunosuppressive factors like transforming growth factor (TGF)-β, IL-10, and indoleamine 2, 3-dioxygenase (IDO) that further limit the effectiveness of NK cells [45]. It’s worth noting that the expression of NK receptors and their cognate ligands on leukemic cells and the signals originating from the tumor microenvironment significantly impact the clinical outcomes and relapse [46].
Reactive oxygen species (ROS) can trigger the expression of the poliovirus receptor (PVR), recognized by DNAM-1 and nectin-2 in many cancers [47]. DNAM-1 can collaborate with other NK cell receptors, such as NKp30, NKp44, and NKp46, to mediate tumor cell killing, including AML cells [48]. Despite identifying ligands for NKp46, B7-H6 and MLL5 can be expressed on AML blasts. Other receptors, such as lymphocyte function-associated antigen-1 (LFA-1) and signaling lymphocytic activation molecule (SLAM) family receptors, can induce NK cell activation in cell-to-cell interactions with targets. LFA-1 binds to intercellular adhesion molecules-1 (ICAM-1 or CD54) on most AML cells, while SLAM receptors involve homotypic interactions except for 2B4, which recognizes CD48 [49, 50].
Role of NK receptors, KIR and other receptors in CLL
Multiple studies have shown that CLL patients have reduced expression of various activating receptors, like NKp30, NKp46, NKG2D, and DNAM-1, compared to healthy donors and associated with poor prognostic factors [51]. The expression of NKG2D on CLL-derived NK cells reduces significantly in advanced and progressive disease, showing down regulation by CLL cells. A decrease in the expression of NKG2D, lowered cytotoxicity of NK cells, and reduced production of IFNγ. This indicates that CLL cells can alter the functions of NK cells, which leads to a state of hypo-responsiveness. The chronic lymphocytic leukemia (CLL) cells may release high amount of TGFβ, which leads to the down regulation of NKG2D expression on NK cells, hence impairs the activity of NK cells [52]. Leukemic cells in CLL have low expression of ligands for NK cell activating receptors, which negatively affects the activity of NK cells [53]. This common expression is primarily due to the shedding of released ligands as soluble molecules, inhibiting tumor cell recognition by NK cells, representing an escape mechanism seen in many cancers [54, 55].
It has been found that CD94/NKG2A, can reduce NK cell cytotoxicity against CLL cells by binding to HLA-E molecules. These molecules are highly expressed on the CLL cell surface HLA-E binding to CD94/NKG2A can lead to signals that suppress cytokine secretion and direct cytotoxicity of effector cells against cancerous cells, contributing to tumor escape [56, 57].
The inhibitory KIRs in NK cells from CLL patients have similar expression levels of KIRDL2/3 and KIRDL1as healthy individuals, remaining stable even during disease progression. However, in patients with unfavorable prognosis, there is a slight decrease in KIR2DL1 expression, which recognizes group-2 HLA-C alleles. Impaired NK cell activity in CLL leads to a significant reduction in KIR2DL1 and KIR3DL1 expression. Throughout the disease, these cells lose their functions and undergo activation-induced apoptosis, promoting the expansion of nonfunctional NK cells [58, 59, and 60].
Studies have shown that natural killer (NK) cells from patients with chronic lymphocytic leukemia (CLL) tend to over-express the ILT2/CD85j inhibitory receptor, particularly in advanced stages. On the other hand, CLL cells abnormally express the ligand HLA-G, which is associated with poor prognosis and suppresses NK cell-mediated cytotoxicity [61].
Interestingly CLL cells use different mechanisms to adopt NK cell immune surveillance. The tumor cells release ligands for activating receptors on NK cells through proteolytic shedding from their surface [62]. The mechanism involved in the CLL and how the immune cells are escaped is shown in Figure-4.
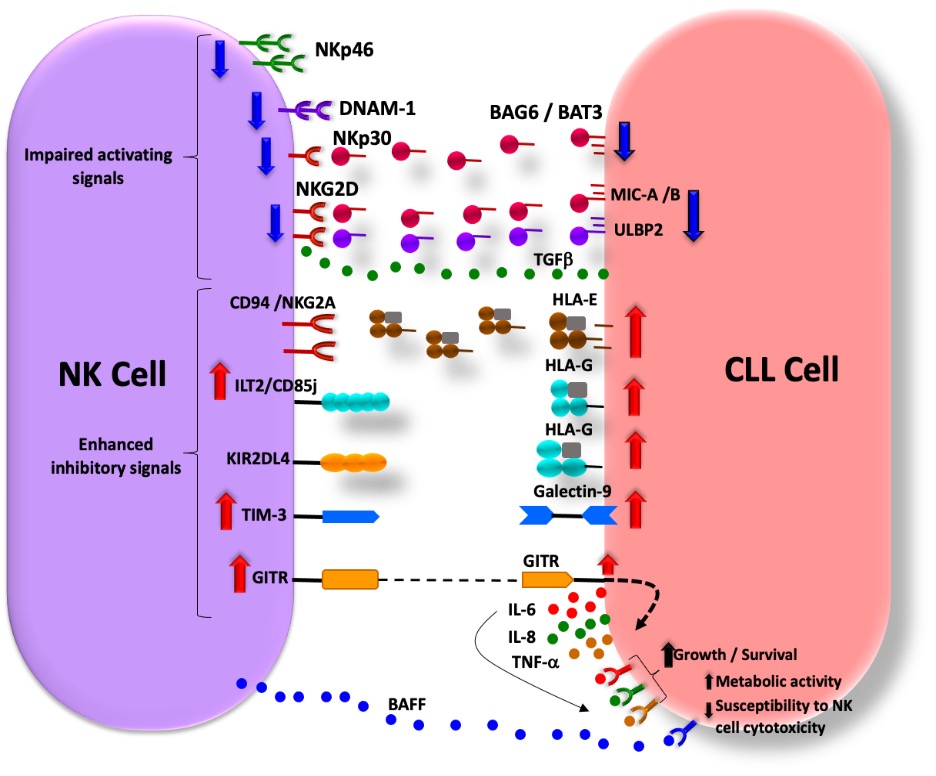
Figure 4: Mechanism of immune Immune surveillance in CLL. (i) reduced expression of activating receptors on NK cells or their ligands on CLL cells; (ii) release of NK cell activating receptor ligands by CLL cells; (iii) increased expression of inhibitory receptors on NK cell surfaces and their ligands on CLL cells; (iv) NK cell-induced signals that promote CLL cell growth, survival, and metabolic activity while hindering CLL cell susceptibility to NK cell-mediated cytotoxicity. The red and blue arrows indicate increased and decreased cell surface expression, respectively, of NK cell receptors or their ligands on CLL cells. The dotted line indicates GITR-GITRL interaction, while dotted arrows indicate intracellular signaling.
Reinerset.al, demonstrated that the soluble NKp30 ligand BAG6/BAT3, which is detectable in the plasma of patients with chronic lymphocytic leukemia (CLL), suppresses the cytotoxicity of natural killer (NK) cells. Additionally, BAG6/BAT3 down regulates the expression of CD16 and CD56 on NK cells from healthy donors [63]. They have further shown that BAG6 can activate NK cell cytotoxicity when it’s expressed on the surface of exosomes. This suggests that exosomal BAG6 can play a role in the “induced self-activation” of NK cells. Moreover, a disrupted balance between exosomal and soluble BAG6 expression might enable CLL to evade NK cells. The plasma of patients with chronic lymphocytic leukemia (CLL) contains higher levels of various factors that can compromise the function of natural killer (NK) cells. These factors include macrophage migration inhibitory factor (MIF), soluble NKG2D ligands MIC-B and ULBP2. In patients with advanced stages of the disease, the levels of soluble BAG6/BAT3, MIC-B, and ULBP2 are further increased, thus indicating their potential role as prognostic factors. Studies have confirmed the prognostic significance of soluble MIC-A, MIC-B, and ULBP2 in CLL. Among these ligands, soluble ULBP2 is the most important prognostic marker, which can identify early-stage patients at risk of disease progression [64].
NK cell receptors and CML
The NK cell number is down regulated in CML cases as the disease progresses from the chronic phase to blast crisis; additionally, NK cells isolated from patients at an advanced stage of the disease show reduced cytotoxicity and reduced anti -tumor response. [64] The expression of activating receptors NKp30, NKp46, NKG2A, NKG2C, and NKG2D are down regulated in CML cases. In CML, translocation of the BCR/ABL gene affects dendritic cells, enabling them to activate NK cells by increasing the expression of NKG2D ligands [65]. The BCR-ABL gene directly controls the expression of NKG2DL and the antitumor reactivity of the NK cells is directly dependent on the quantity of NKG2DL on the cellular surface with MICA being the most expressed [66]. Hence in CML, it is by means of the NKG2D/MICA interaction that NK cells exercise their cytotoxic role against tumor cells. In chronic exposure of NKG2D, its MICA ligand can secrete soluble proteins produced on the surface of tumor cells, denominated sMICA. [67] Increased serum levels of sMICA reflect tumor expansion, since healthy tissues present significantly lower levels of sMICA (23). The release of sMICA with chronic exposure of NKG2DL expressed in tumor cells, induces a negative modulation of the NKG2D that remains on the surface of NK cells of patients with CML and other types of cancer, facilitating the escape of tumor cells from lysis mediated by NK cells (36). Blocking of sMICA production may be an important clinical strategy for an antitumor response [68].
KIR and CML
Various mechanisms are involved: shedding of ligands by leukemic cells, down regulation of activating receptors, expansion of myeloid‐derived suppressor cells that promotes the recruitment of Treg and impairs NK cells in a membrane‐bound TGFβ1 manner [69, 70]. The receptor KIR2DL5 (KIR2DL5A and KIR2DL5B) belongs to KIR receptor family and possesses a unique combination of genetic, structural, and functional hallmarks that confer an inhibitory function when binding to its unknown ligand [71]. In the setting of CML, KIR2DL5A and KIR2DL5B genotypes have been associated with a decrease in the rate of 12‐month molecular response and 2‐year complete cytogenetic response [72]. A specific KIR2DL5B genotype has been shown to predict a bad prognosis through various outcomes in CML in a first‐line imatinib strategy, including transformation‐free survival, suggesting its important role in CML immune escape. [73]
Immunotherapy in AML, ALL, CML and CLL
The non-traditional HLA antigens mentioned above have been conclusively proven to hinder the ability of NK cells to eliminate target cells, restrict their proliferation, and reduce their capacity to cross blood vessels. These antigens also obstruct the functions of cytolytic T cells, B cells, and dendritic cells. Besides KIR2DL4, HLA-G binds to several other inhibitory receptors, including ILT2/CD58j, ILT4, and CD160, which are present in many immune cells. Patients with CLL have been found to have significantly elevated levels of soluble HLA-G, and this has been firmly established. Adoptive immunotherapy is a form of cancer treatment that involves the usage of natural killer (NK) cells to kill tumors. NK cells can come from patients, healthy donors, or autologous or allogeneic sources. The effectiveness of the treatment depends on the quality of the NK cells used. Currently, only autologous NK cells are used in cancer immunotherapy.
AML: Monoclonal antibodies (mAbs) have been developed to target tumor antigens and are currently used in clinical settings. Several mechanisms of action have been identified, including recognizing the Fc part of human or humanized IgG1 or IgG3 isotypes by CD16, which NK and myeloid cells express. When CD16 is engaged, the cells are activated and eliminate the targeted cells. Studies showed that monoclonal anti-CD123 antibody improved the binding to CD16a and enhanced the anti-leukemic activity of NK cells against AML xenograft models [74]. Further, Koerner et al. found that an Fc-optimized CD133 antibody had a greater affinity to NK cells and more cytotoxic activity for NK cells without relevant toxicity to hematopoietic progenitors in a human AML xenograft model [75]. Bispecific killer cell engagers (BiKEs) are engineered antibodies with dual specificity for tumor antigens like CD19 or CD20 in B-cell-related diseases and CD16 targeting NK cells. The anti-CD16 part of theBikesreceive as potent therapeutic tools for AML and other cancers [76]. However, the use of BiKEs in AML treatment remains limited due to tumor heterogeneity in AML patients.
The up regulation of HLA-E on AML blasts when activated by IFN-gamma impairs CD94. This impairment is likely to prevent the activation of NK cells and lead to NK-mediated immune evasion. Currently, blocking antibodies are being assessed to inhibit these mechanisms. The monoclonal antibody with plate number 1 has been considered to block KIR-ligand interaction [77]. In vitro studies demonstrated thata novel anti-inhibitory KIR antibody (IPH2101) increases NK cell-mediated lysis of KIR-ligand matched tumor cells and enhances NK cell-mediated ADCC against antibody-bound tumors [78]. Lirilumab is currently undergoing testing in patients who are in complete remission for long-term maintenance (#NCT01687387), and for the treatment of patients with refractory/relapsed AML (#NCT02399917) [79]. Additionally, lirilumab also recognizes KIR2DS1 and KIR2DS2; blocking these receptors may, on the other hand, hinder tumor cell clearance [80]. As AML cells are expected to express HLA-E, it is plausible that this new antibody may also be effective in treating AML. Other inhibitory receptors, such as PD-1, LAG-3, or TIM-3, have been classified as "inhibitory checkpoint receptors" and could also affect the activity of NK cells. PD-1 was expressed in NK cells, which led to reduced expression of NKp46 and NKG2D, impaired NK cell function, and decreased secretion of IFN-γ. However, blocking the PD-1 inhibitory pathway could restore IFN-γ secretion [81]. IFN-γ can induce the expression of PD-L1 on AML cells, leading to the inhibition of the antileukemic response mediated by T-lymphocytes and NK cells [82]. Therefore, the anti-PD-1 mAb Nivolumab is tested in a phase II clinical trial in AML patients in remission with a high risk of relapse (#NCT02532231).
ALL: Various monoclonal antibodies have been designed to attack a specific target, such as a protein on the surface of leukemia cells, e.g., Blinatumomab, aparticular monoclonal antibody because it can attach to 2 different proteins simultaneously. One part of blinatumomab binds to the CD19 protein found on B cells, including some leukemia and lymphoma cells. Another part attaches to CD3, a protein in immune cells called T cells. By binding to both proteins, this drug brings the cancer cells and immune cells together, which is thought to cause the immune system to attack the cancer cells. This drug is used to treat some types of B-cells ALL. Inotuzumab ozogamicinis is an anti-CD22 antibody linked to a chemotherapy drug. B cells, including some leukemia cells, usually have the CD22 protein on their surface. The antibody acts like a homing signal, bringing the chemo drug to the leukemia cells, entering them, and killing them when they divide into new cells. It is used to treat some types of B-cells ALL, typically after chemotherapy has been tried.
CLL: Immunotherapy is a treatment option for CLL that involves the use of tumor-specific monoclonal antibodies (mAbs), bi-specific, and tri-specific killer engagers (BiKEs or TriKEs). Rituximab, a monoclonal antibody, is used to target CD20, which induces complement-dependent cytotoxicity (CDC) (Figure-5)
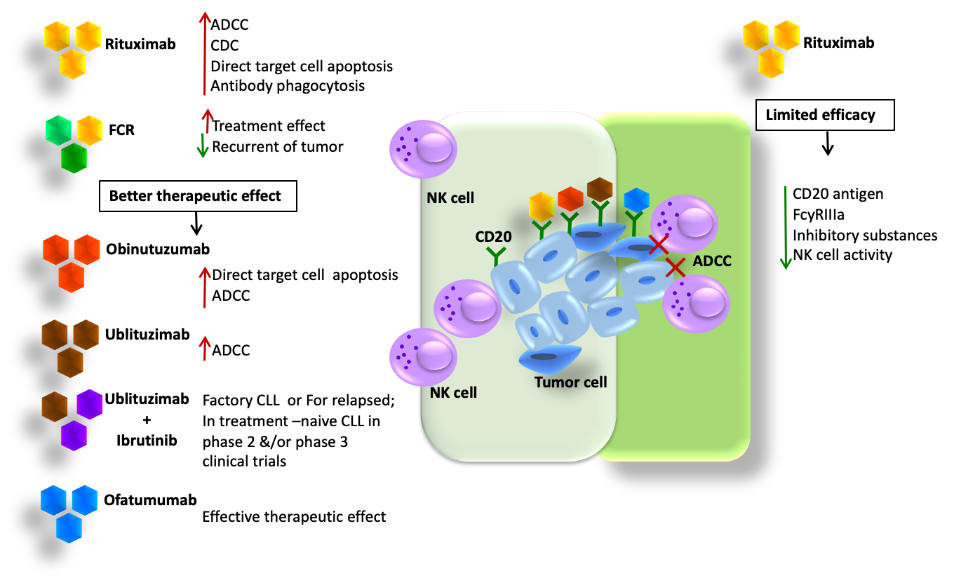
Figure 5: The antitumor mechanisms of anti-CD20 mAbs
Directs target cell apoptosis, and antibody-dependent phagocytosis. However, the efficacy of CD20 is limited because it is susceptible to the loss of CD20 antigen, which causes an increase in antigen-loss cells that are resistant to NK cell-mediated ADCC, resulting in reduced efficacy. Rituximab has poor affinity to FcgRIIIa and weakens NK cell-mediated
ADCC by increasing the release of inhibitory substances that suppress the immune response [83]. In CLL patients, NK cell activity is impaired. Other antibodies like Obinutuzumab, Ofatumumab, and Ublituximab help in glycol engineering. Inebilizumab (MEDI-551) targets CD19 and increases the binding affinity of mAbs to FcgRIIIa. CD16 targets the BiKEs, which are Bi-specific and tri-specific killer cell are composed of a single-chain variable fragment (scFv) recognizing CD16 and a scFv recognizing tumor antigens. This helps in revealing the transferring signals as immunological synapses and inducing cytotoxicity. There are limited studies on therapeutic strategies exploiting BiKEs and TriKEs to trigger NK cells in CLL, but the results suggest that they are promising in NK cell immunotherapy in CLL.
Another approach in allogeneic immunotherapy is the use of BCR and Bcl-2 inhibitors in treating high-risk CLL, allogeneic hematopoietic stem cell transplantation (allo-HSCT) for recurrent or refractory CLL [84]. These inhibitors are associated with both risks and benefits of allo-HSCT in high-risk CLL [85].
CML: Some studies have included various immunotherapeutic approaches for CML by targeting the underlying pathways, such as the programmed cell death protein 1 (PD‑1)/PD‑1 ligand 1 (PDL‑1), interleukin (IL) ‑1 and JAK/STAT pathways [86]. The PD-1/PDL-1 interaction may act as an immune checkpoint inhibitor, having a marked effect on the anti‑tumor response. In patients with CML, PD‑1 is highly expressed on CML‑specific CTLs, and PD‑1/PDL‑1 interaction leads to the exhaustion and inhibition of CTLs in patients with CML. Therefore, blocking PD‑1/PDL‑1 binding as a treatment strategy represents a promising therapeutic approach for CML [87].
IL‑1 is a regulator of inflammation and the innate immune response, having numerous roles in immunopathological functions. In CML, since IL‑1 provides resistance to imatinib in CML, the prevention of IL-1 signaling has the potential to increase the efficacy of TKI treatment. Additionally, when using IFN‑αtreatment in CML, IFN‑α inhibits IL‑1 due to its anti‑inflammatory effect. Hence, a combination therapy with TKIs and IFN-α may be a more efficacious approach for CML patients [88]. Meanwhile, IFN‑α‑resistant CML patients have high levels of IL-1, which stimulates IFN-α‑sensitive CML cells. One way of the blocking IL‑1 pathway is using IL‑1 receptor accessory (IL1RAP) ‑specific chimeric antigen receptor‑modified T (CAR-T) cells. IL1RAP-CAR-T cells represent an important alternative therapy for patients with CML presenting with TKI and IFN‑α resistance [89].
Therapeutic antibodies can achieve anti-tumor responses by modulating the activity of their protein targets and by redirecting effector cells of the immune system to the cancer cells. By targeting cell surface proteins up regulated on the malignant cells, a selective immune response can be activated against the cancer cells. This is an essential area of research in cancer treatment and could lead to the development of more effective cancer therapies in the future [90].
Allo-reactive NK cells from transplantation to adoptive immunotherapy
Adoptive transfer allogeneic NK cells have emerged as promising immunotherapy for hematological malignancies [91]. The role of alloreactive NK cells is considered to be beneficial in achieving better outcome of haploidentical HSCT (haplo-HSCT). Haplo-HSCT can lead to graft versus host disease (GvHD), this problem has been currently solved by performing of T cell depletion before graft infusion. After chemotherapy in AML patients, T cell prophylaxis has been used together with high stem cell doses, resulting in fast NK cell alloreactivities and slow T cell reconstitution. Moreover, graft versus leukemic cells mediated by NK cell, allo-reactivities have been exploited which they can beneficially lead to reduced relapse, and improve survival [92]. KIR-ligand mismatches between donor and recipient under haplo HSCT setting, allo-reactive NK cells play crucial roles against leukemic cells, recipients’ DCs and T cells, resulting in reduced leukemic relapses, GvHD and graft rejection, respectively [93].
Additionally, four situations in predicting NK cell allo-reactivities after haplo-HSCT have been proposed based on the deference in definition of KIR mismatches between the donor NK cells and the recipient’s HLA [94].
AML and KIR/KIR-ligand mismatches hematopoietic stem cell transplantation
Babor et al. have shown that there is growing evidence that KIRB genotyped donors can have a positive impact on hematopoietic stem cell transplantation (HSCT) for acute myeloid leukemia (AML) [95]. The "missing self" theory suggests that the lack of cognate ligands for inhibitory KIRs allows AML cells to activate NK cells, eliminating the leukemic target. [36] This theory is supported by the observation that activating KIR2DS1 can significantly reduce AML relapse in donor/recipient pairs where the recipient expresses specific HLA-C ligands [68]. Based on these results, selecting the right donor may be crucial to optimize the graft-vs.-leukemia effect expected from the HSC transplantation.Interestingly, natural killer (NK) cells recognize and eliminate target cells through a process of "missing self" and "induced self" recognition [96]. During NK cell development, inhibitory KIR encounters MHC class I (MHC-I) ligands on hematopoietic cells leading to the acquisition of functional competence and self-tolerance. Reduction/absence of MHC-I molecules and upregulation/de novo expression of ligands for activating receptors on tumor cells can elicit NK cell immune response against “non-self”. This response involves releasing cytotoxic granules, secreting cytokines, and inducing death receptor-dependent apoptosis [97].
NK cell-based immunotherapy in ALL, AML, CLL and CML
NK cell-based immunotherapy is a promising treatment option for combatting acute myeloid leukemia (AML) [98]. Allo-reactivity mediated by donor NK cells can kill leukemia through the graft-versus-leukemia (GvL) effect, promote engraftment by removing recipient T cells, and protect against graft-versus-host disease (GvHD) by depleting recipient antigen-presenting cells and producing IL-10 [99, 100]
Allogeneic hematopoietic cell transplantation (allo-HCT) recipients have a better chance of survival when transplanted with NK allo-reactive donors, especially if the donors have more KIR B gene content motifs (101). Additionally, rapid NK cell recovery following HCT is associated with improved outcomes, while impaired NK function may lead to relapse [102, 103]
In summary, given the fundamental concepts of NK cell allo-reactivity and the predictive effects of functional NK cell counts, adoptive transfer of NK cells could be a valuable approach for treating AML management of AML. Immunotherapy is a successful option, but still, a lot of transplant-related mortality and morbidity is associated with these patients. Therefore, adoptive NK cell transfer seems to be an ideal option as an adjuvant and alternative treatment, and it has already been performed in the context of HCT and the non-HCT setting. Adoptive transfer of natural killer (NK) cells has emerged as a promising option for cellular immunotherapy to treat acute myeloid leukemia (AML).
There are several advantages of using NK cells in AML treatment. They can target cancer cells without prior sensitization, have a low risk of causing graft-versus-host disease (GVHD), and induce long-term remission (104). Immunomodulatory imide drugs (IMiDs) are a class of drugs that adjust immune responses containing an imide group. This class includes thalidomide and its analogues lenalidomide, pomalidomide, and iberdomide. They increase the expression of activating receptors, notably NCR [105]. Immuno-monitoring studies in several clinical trials confirmed the expansion of NK cells by these molecules [106, 107].
IL-2 produced by T cells mediates NK cell ADCC. Additionally, it has been shown that lenalidomide improves the stability of the immune synapse, enhancing tumor cell recognition by NK cells [108].
CAR -NK-Cell therapy
Adoptive NK cell transfer has proven to be an effective strategy in fighting against AML cells, with the success of this approach largely dependent on the interactions between activating and inhibitory receptors of NK cells and their corresponding ligands present on the target cells. To further enhance specificity and cytotoxicity (109). Scientists have designed genetically modified NK cells, such as CAR-modified-NK cells. (Figure-6).
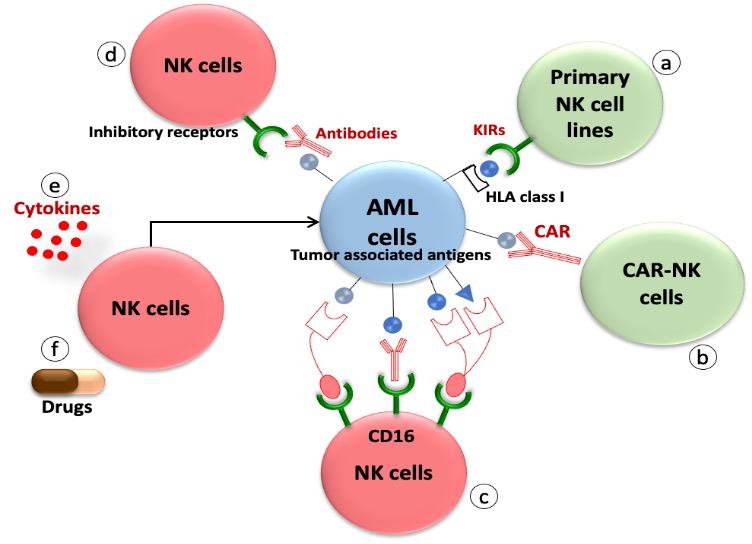
Figure 6: Strategies of NK cell-based immunotherapy in activating the reconstitution of NK cells against AML.
However, unlike CAR-T therapy, which has successfully treated B-lineage acute lymphoblastic leukemia and B-cell lymphoma, its application is limited in AML due to its adverse effects (110).
These cells are designed to address two key aspects of cancer treatment - improving efficacy and controlling adverse effects such as acute cytokine release syndrome (CRS), neurotoxicity, and graft-versus-host disease (GVHD). These cells target and destroy cancer cells through a combination of NK cell receptor-dependent and CAR-dependent signaling cascades. Overall, CAR-NK cells offer a promising and effective approach to cancer treatment, thanks to their ability to simultaneously improve outcomes and manage adverse effects (70). There are some challenges in using CAR-NK cells in treating acute myeloid leukemia (AML), such as selecting leukemia-specific markers, there are promising targets that can be used, such as CD33, CD4, and CD7 (111)
In Soldierer et al. recent paper (71) demonstrate the role of CAR-NK cell therapy in treating ALL and AML cases. NK cells can be transplanted across HLA barriers without causing graft-versus-host disease. This makes them a viable option for off-the-shelf usage to expand clinical indications and limit treatment costs per patient. Furthermore, the researchers modified CAR constructs by recognizing standard target antigens for acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) therapy, CD33, and CD123 to harbor a CD34-derived hinge region. This allows efficient detection of transduced NK cells in vitro and in vivo and also facilitates CD34 microbead-assisted selection of CAR NK cell products to >95% purity for potential clinical usage. The researchers have developed an in vitro system that blocks activating receptors NKG2D, DNAM-1, NKp30, NKp44, NKp46, and NKp80 on these cells for testing the specific killing of CAR NK cells against ALL and AML cell lines and primary AML blasts. Finally, the researchers evaluated in an ALL-xenotransplantation model in NOD/SCID-gamma (NSG) mice whether human CD19 CAR NK cells directed against the CD19+ blasts rely on soluble or membrane-bound IL15 production for NK cell persistence and also in vivo leukemia control. This study provides critical insights into generating pure and highly active allogeneic CAR NK cells, further advancing adoptive cellular immunotherapy with CAR NK cells for human malignancies (70).
Summary
NK cells are a crucial part of the immune response against cancer, especially in haplo HSCT treatment for high-risk leukemia in adults and children. The anti-leukemic effect is primarily due to the presence of "alloreactive" NK cells. Certain activating KIR, particularly KIR2DS1, also play an important role when they interact with their HLA class I ligand (C2 alleles). The recent haplo HSCT method (depletion of αβ T and CD19+ B cells) allows the infusion of mature NK cells and γδ T cells, in addition to high doses of donor CD34+ HSCs, which helps control leukemia recurrence after haplo HSCT. Small pilot trials investigating NK adoptive transfer have shown that this approach is safe and feasible, particularly with highly pure infusions of NK cells. However, the efficacy of this approach is yet to be established due to small sample sizes in all of these studies. In most of these studies, survival rates did not appear to be substantially different from outcomes without NK adoptive therapy. Larger randomized trials are necessary to establish the efficacy of NK adoptive immuno-therapy. Additionally, several questions remain unanswered, including the best timing of NK adoptive transfer, ideal disease targets and disease states, preparative regimens for adoptive transfer if not in the immediate post-HSCT setting, and the best methods to enhance NK number and activity. The studies on NK cell development and function have led to the development of new NK-based immunotherapies, such as the use of bi-specific/tri-specific mAbs linking NK cells to target antigens and/or cytokines, fully human anti-KIR mAb, and NK cells engineered to express a chimeric antigen receptor (CAR) specific for surface tumor antigens. These novel approaches have the potential to revolutionize the field and can be applied in both HSCT and NK adoptive transfer settings.
Acknowledgement
The authors are thankful to Sanjay Kumar Johari for his official assistance in preparation of this manuscript at all steps.
References
- Morris, P.J.; Batchelor, J.R.; Festenstein, H. )1978). Matching for HLA in transplantation. Br. Med. Bull., 34, 259-262.
View at Publisher | View at Google Scholar - Saeki, Y.; Ishiyama, K.; Ishida, N.; Tanaka, Y.; Ohdan, H. (2017). Role of Natural Killer Cells in the Innate Immune System After Intraportal Islet Transplantation in Mice. Transplant. Proc., 49, 139-144.
View at Publisher | View at Google Scholar - Lin, C.M.; Gill, R.G.; Mehrad, B. (2021). The natural killer cell activating receptor, NKG2D, is critical to antibody-dependent chronic rejection in heart transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg.
View at Publisher | View at Google Scholar - López-Botet, M.; Vilches, C.; Redondo-Pachón, D.; Muntasell, A.; Pupuleku, A.; Yélamos, J.; Pascual, J.; Crespo, M. (2017). Dual Role of Natural Killer Cells on Graft Rejection and Control of Cytomegalovirus Infection in Renal Transplantation. Front. Immunol., 8, 166.
View at Publisher | View at Google Scholar - Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. (2008) Functions of natural killer cells. Nat Immunol. 9:503-510.
View at Publisher | View at Google Scholar - Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. (2006). Human NK cell education by inhibitory receptors for MHC class I. Immunity. 25:331-342.
View at Publisher | View at Google Scholar - Bryceson YT, Ljunggren HG, Long EO. (2009) The minimal requirement for induction of natural cytotoxicity and the intersection of activation signals by inhibitory receptors. Blood. 114:2657-2666.
View at Publisher | View at Google Scholar - Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. (2005). Different receptors in resting NK cells control cytolytic granule polarization and degranulation. J Exp Med. 202:1001-1012.
View at Publisher | View at Google Scholar - Screpanti V, Wallin RP, Ljunggren HG, Grandien A. (2001). A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J Immunol. 167:2068-2073.
View at Publisher | View at Google Scholar - Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. (1998) 188:2375-2380.
View at Publisher | View at Google Scholar - Iyori M, Zhang T, Pantel H, Gagne BA, Sentman CL. (2011). TRAIL/DR5 plays a critical role in NK cell-mediated negative regulation of dendritic cell cross-priming of T cells. J Immunol. 187:3087-3095.
View at Publisher | View at Google Scholar - Herberman RB, Nunn ME, Holden HT, Lavrin DH. (1975) Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 16:230-239.
View at Publisher | View at Google Scholar - Alspach E, Lussier DM, Schreiber RD. (2019). Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb Perspect Biol. Mar 1;11(3): 028480.
View at Publisher | View at Google Scholar - Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, et al. (2012) Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 119:2665-2674.
View at Publisher | View at Google Scholar - Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. (2012) 189:5082-5088.
View at Publisher | View at Google Scholar - Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. (2004) 5:996-1002.
View at Publisher | View at Google Scholar - Horowitz A, Djaoud Z, Nemat-Gorgani N, Blokhuis J, Hilton HG, Béziat V, (2016). Malmberg KJ, Norman PJ, Guethlein LA, Parham P. Class I HLA haplotypes form two schools that educate NK cells in different ways. Sci Immunol. Sep;1(3): eaag1672.
View at Publisher | View at Google Scholar - Wilson MJ, Torkar M, Haude A, et al. Plasticity in the organization and sequences of human KIR/ILT gene families. ProcNatlAcadSci U S A 2000; 97:4778-83.
View at Publisher | View at Google Scholar - Uhrberg M. The KIR gene family: life in the fast lane of evolution. Eur J Immunol 2005: 35: 10-15.
View at Publisher | View at Google Scholar - Uhrberg M, Valiante NM, Shum BP, Shilling HG, Lienert-Weidenbach K, Corliss B, Tyan D, Lanier LL, Parham P. (1997). Human diversity in killer cell inhibitory receptor genes. Immunity 7:753;
View at Publisher | View at Google Scholar - MacFarlane AW IV, Campbell KS. Signal transduction in natural killer cells. Curr Top MicrobiolImmunol 2006; 298:23-57.
View at Publisher | View at Google Scholar - Kikuchi-Maki A, Catina TL, Campbell KS. Cutting edge: KIR2DL4 transduces signals into human NK cells through association with the Fc receptor gamma protein. J Immunol 2005; 174:3859-3863.
View at Publisher | View at Google Scholar - Valenzuela-Vázquez L, Nuñez-Enriquez JC, Sánchez-Herrera J, Medina-Sanson A, Pérez-Saldivar ML, Jiménez-Hernández E, Martiín-Trejo JA, Del Campo-Martínez MLÁ, Flores-Lujano J, Amador-Sánchez R, Mora-Ríos FG, Peñaloza-González JG, Duarte-Rodríguez DA, Torres-Nava JR, Espinosa-Elizondo RM, Cortés-Herrera B, Flores-Villegas LV, Merino-Pasaye LE, Almeida-Hernández C, Ramírez-Colorado R, Solís-Labastida KA, Medrano-López F, Pérez-Gómez JA, Velázquez-Aviña MM, Martínez-Ríos A, Aguilar-De Los Santos A, Santillán-Juárez JD, Gurrola-Silva A, García-Velázquez AJ, Mata-Rocha M, Hernández-Echáurregui GA, Sepúlveda-Robles OA, Rosas-Vargas H, Mancilla-Herrera I, Jimenez-Morales S, Hidalgo-Miranda A, Martinez-Duncker I, Waight JD, Hance KW, Madauss KP, Mejía-Aranguré JM, Cruz-Munoz ME. NK cells with decreased expression of multiple activating receptors are a dominant phenotype in pediatric patients with acute lymphoblastic leukemia. Front Oncol. 2022 Nov 7; 12:1023510.
View at Publisher | View at Google Scholar - Mizia-Malarz A., Sobol-Milejska G. (2019). NK cells as a possible prognostic factor in childhood acute lymphoblastic leukemia. Disease Markers.; 2019:13.
View at Publisher | View at Google Scholar - 24- Babor F, Manser AR, Fischer JC, Scherenschlich N, Enczmann J, Chazara O, Moffett A, Borkhardt A, Meisel R, Uhrberg M. KIR ligand C2 is associated with increased susceptibility to childhood ALL and confers an elevated risk for late relapse. Blood. 2014 Oct 2;124(14):2248-2251.
View at Publisher | View at Google Scholar - Bachir F., Bennani S., Lahjouji A., et al. (2009). Characterization of acute lymphoblastic leukemia subtypes in Moroccan children. International Journal of Pediatrics.; 2009:7.
View at Publisher | View at Google Scholar - Li S. Y., Ye J. Y., Meng F. Y., Li C. F., Yang M. (2015). Clinical characteristics of acute lymphoblastic leukemia in male and female patients: a retrospective analysis of 705 patients. Oncology Letters. ;10(1):453-458.
View at Publisher | View at Google Scholar - Hjalgrim L. L., Rostgaard K., Schmiegelow K., et al. (2003). Age- and sex-specific incidence of childhood leukemia by immunophenotype in the Nordic countries. Journal of the National Cancer Institute. ;95 (20):1539-1544.
View at Publisher | View at Google Scholar - Sathishkumar K, Chaturvedi M, Das P, Stephen S, Mathur P. Cancer incidence estimates for 2022 & projection for 2025: Result from National Cancer Registry Programme, India. Indian J Med Res. 2022 Oct-Nov;156(4&5):598-607.
View at Publisher | View at Google Scholar - Chewning JH, Gudme CN, Hsu KC, Selvakumar A, Dupont B. (2007). KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J Immunol; 179:854-868.
View at Publisher | View at Google Scholar - Woo, J.S.; Alberti, M.O.; Tirado, C.A. Childhood B-acute lymphoblastic leukemia: A genetic update. Exp. Hematol. Oncol. 2014, 3, 16.
View at Publisher | View at Google Scholar - Valenzuela-Vazquez L., Núñez-Enríquez J. C., Sánchez-Herrera J., et al. Functional characterization of NK cells in Mexican pediatric patients with acute lymphoblastic leukemia: report from the Mexican interinstitutional group for identifying the causes of childhood leukemia. PLoS One. 2020;15(1, article e0227314)
View at Publisher | View at Google Scholar - Mizia-Malarz A., Sobol-Milejska G. (2019). NK cells as a possible prognostic factor in childhood acute lymphoblastic leukemia. Disease Markers.
View at Publisher | View at Google Scholar - Liu L. L., Béziat V., Oei V. Y. S., et al. (2017). Ex vivo expanded adaptive NK cells effectively kill primary acute lymphoblastic leukemia cells. Cancer Immunology Research. ;5(8):654-665.
View at Publisher | View at Google Scholar - Zhang C, Wang XM, Li SR, Twelkmeyer T, Wang WH, Zhang SY, Wang SF, Chen JZ, Jin X, Wu YZ, Chen XW, Wang SD, Niu JQ, Chen HR, (2019). Tang H. NKG2A is an NK cell exhaustion checkpoint for HCV persistence. Nat Commun. Apr 3;10(1):1507.
View at Publisher | View at Google Scholar - Bi J, Tian Z. (2017). NK Cell Exhaustion. Front Immunol. Jun 28; 8:760.
View at Publisher | View at Google Scholar - Duault C, Kumar A, Taghi Khani A, Lee SJ, Yang L, Huang M, Hurtz C, Manning B, Ghoda L, McDonald T, Lacayo NJ, Sakamoto KM, Carroll M, Tasian SK, Marcucci G, Yu J, Caligiuri MA, Maecker HT, Swaminathan S.at all. (2021). Activated natural killer cells predict poor clinical prognosis in high-risk B- and T-cell acute lymphoblastic leukemia. Blood. Oct 21;138(16):1465-1480.
View at Publisher | View at Google Scholar - Shindo T., Ureshino H., Kojima H., Tanaka H., Kimura S. (2021). Allelic polymorphisms of KIRs and antitumor immunity against chronic myeloid leukemia. Immunol Med. ;44(2):61-68.
View at Publisher | View at Google Scholar - Zhang Y., Wang B., Ye S., et al. (2010). Killer cell immunoglobulin-like receptor gene polymorphisms in patients with leukemia: possible association with susceptibility to the disease. Leukemia Research. ;34(1):55-58.
View at Publisher | View at Google Scholar - Almalte Z., Samarani S., Iannello A., et al. (2011). Novel associations between activating killer-cell immunoglobulin-like receptor genes and childhood leukemia. Blood. ;118(5):1323-1328.
View at Publisher | View at Google Scholar - Nowak I., Płoski R., Barcz E., et al. (2015). KIR2DS5, in the presence of HLA-C C2, protects against endometriosis. Immunogenetics. ;67(4):203-209.
View at Publisher | View at Google Scholar - Augusto D. G. (2016). The impact of KIR polymorphism on the risk of developing cancer: not as strong as imagined? 7:1-9.
View at Publisher | View at Google Scholar - Babor F., Manser A. R., Fischer J. C., et al. (2014). KIR ligand C2 is associated with increased susceptibility to childhood ALL and confers an elevated risk for late relapse. Blood. ;124(14):2248-2251.
View at Publisher | View at Google Scholar - Hurabielle C., Thonnart N., Ram-Wolff C., et al. (2017). Usefulness of KIR3DL2 to diagnose, follow-up, and manage the treatment of patients with Sézary syndrome. Clinical Cancer Research. ;23(14):3619-3627.
View at Publisher | View at Google Scholar - Costello R. T., Sivori S., Marcenaro E., et al. (2010). Lack of expression of inhibitory KIR3DL1 receptor in patients with natural killer cell-type lymphoproliferative disease of granular lymphocytes. Haematologica . ;95(10):1722-1729.
View at Publisher | View at Google Scholar - Hikami K., Tsuchiya N., Yabe T., Tokunaga K. Variations of human killer cell lectin-like receptors: common occurrence of NKG2-C deletion in the general population. Genes and Immunity. 2003;4(2):160-167.
View at Publisher | View at Google Scholar - Davidson C. L., Li N. L., Burshtyn D. N. (2010). LILRB1 polymorphism and surface phenotypes of natural killer cells. Human Immunology. ;71(10):942-949.
View at Publisher | View at Google Scholar - Kang X., Kim J., Deng M., et al. (2016). Inhibitory leukocyte immunoglobulin-like receptors: immune checkpoint proteins and tumor sustaining factors. Cell Cycle. ;15(1):25-40.
View at Publisher | View at Google Scholar - Machuldova A., Holubova M., Caputo V. S., et al. (2021). Role of polymorphisms of NKG2D receptor and its ligands in acute myeloid leukemia and human stem cell transplantation. Frontiers in Immunology ;12
View at Publisher | View at Google Scholar - Forbes S. A., Beare D., Gunasekaran P., et al. (2015). COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Research. ;43(D1):805-811.
View at Publisher | View at Google Scholar - Linsley P. S., Speake C., Whalen E., Chaussabel D. Copy number loss of the interferon gene cluster in melanomas is linked to reduced T cell infiltrate and poor patient prognosis. PLoS One. 2014;9.
View at Publisher | View at Google Scholar - Orr M. T., Wu J., Fang M., et al. (2010). Development and function of CD94-deficient natural killer cells. PLoS One. ;5(12, article e15184)
View at Publisher | View at Google Scholar - Notario L., Alari-Pahissa E., De Molina A., Lauzurica P. (2016). CD69 deficiency enhances the host response to vaccinia virus infection through altered NK cell homeostasis. Journal of Virology. ;90(14):6464-6474.
View at Publisher | View at Google Scholar - Henrichsen C. N., Vinckenbosch N., Zöllner S., et al. (2009). Segmental copy number variation shapes tissue transcriptomes. Nature Genetics. ;41(4):424-429.
View at Publisher | View at Google Scholar - Gao X., Lin J., Wang L., Yu L. Demethylating treatment suppresses natural killer cell cytolytic activity. Molecular Immunology. 2009;46(10):2064-2070.
View at Publisher | View at Google Scholar - Nugent B. M., Wright C. L., Shetty A. C., et al. Brain feminization requires active repression of masculinization via DNA methylation. Nature Neuroscience. 2015;18(5):690-697.
View at Publisher | View at Google Scholar - Santourlidis S., Trompeter H.-I., Weinhold S., et al. Crucial role of DNA methylation in determination of clonally distributed killer cell Ig-like receptor expression patterns in NK cells. Journal of Immunology. 2002;169(8):4253-4261.
View at Publisher | View at Google Scholar - Costello R. T., Sivori S., Marcenaro E., et al. (2010). Lack of expression of inhibitory KIR3DL1 receptor in patients with natural killer cell-type lymphoproliferative disease of granular lymphocytes. Haematologica . ;95(10):1722-1729.
View at Publisher | View at Google Scholar - Li G., Weyand C. M., Goronzy J. J. Epigenetic mechanisms of age-dependent KIR2DL4 expression in T cells. Journal of Leukocyte Biology. 2008;84(3):824-834.
View at Publisher | View at Google Scholar - Sivori S., Pende D., Bottino C., et al. (1999). NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. European Journal of Immunology. ;29(5):1656-1666.
View at Publisher | View at Google Scholar - Stringaris K., Sekine T., Khoder A., et al. (2014). Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica . ;99(5):836-847.
View at Publisher | View at Google Scholar - Paczulla A. M., Rothfelder K., Raffel S., et al. (2019). Absence of NKG2D ligands defines leukemia stem cells and mediates their immune evasion. Nature. ;572(7768):254–259.
View at Publisher | View at Google Scholar - Reiners K.S., Topolar D., Henke A., Simhadri V.R., Kessler J., Sauer M., Bessler M., Hansen H.P., Tawadros S., Herling M., et al. (2013). Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood.; 121:3658-3665.
View at Publisher | View at Google Scholar - Nückel H., Switala M., Sellmann L., Horn P.A., Dürig J., Dührsen U., Küppers R., Grosse-Wilde H., Rebmann V. (2010). The prognostic significance of soluble NKG2D ligands in B-cell chronic lymphocytic leukemia. Leukemia.; 24:1152-1159.
View at Publisher | View at Google Scholar - Ye Y., Jing Y., Li L., et al. (2020). Sex-associated molecular differences for cancer immunotherapy. Nature Communications. ;11(1). 1779.
View at Publisher | View at Google Scholar - Marcotte, E.L.; Spector, L.G.; Mendes-de-Almeida, D.P.; Nelson, H.H. (2021). The Prenatal Origin of Childhood Leukemia: Potential Applications for Epidemiology and Newborn Screening. Front. Pediatrics, 9, 639479.
View at Publisher | View at Google Scholar - Marinescu, C.; Vlădăreanu, A.M.; Mihai, F. (2015). Acute Lymphocytic Leukemia in Adults. Pathologic Features and Prognosis. Rom. J. Intern. Med., 53, 31-36.
View at Publisher | View at Google Scholar - Childhood Acute Lymphoblastic Leukemia Treatment. Available online: Mizia-Malarz, A.; Sobol-Milejska, G. (2019). NK Cells as Possible Prognostic Factor in Childhood Acute Lymphoblastic Leukemia. Dis. Markers, 3596983.
View at Publisher | View at Google Scholar - Pang Z, Wang Z, Li F, Feng C, Mu X. (2022). Current Progress of CAR-NK Therapy in Cancer Treatment. Cancers (Basel). Sep 2;14(17):4318.
View at Publisher | View at Google Scholar - Soldierer M, Bister A, Haist C, Thivakaran A, Cengiz SC, Sendker S, Bartels N, Thomitzek A, Smorra D, Hejazi M, Uhrberg M, Scheckenbach K, Monzel C, Wiek C, Reinhardt D, Niktoreh N, Hanenberg H.at all, (2022). Genetic Engineering and Enrichment of Human NK Cells for CAR-Enhanced Immunotherapy of Hematological Malignancies. Front Immunol. Apr 7; 13:847008.
View at Publisher | View at Google Scholar - Ferrara, F.; Lessi, F.; Vitagliano, O.; Birkenghi, E.; Rossi, G. (2019). Current Therapeutic Results and Treatment Options for Older Patients with Relapsed Acute Myeloid Leukemia. Cancers, 11, 224.
View at Publisher | View at Google Scholar - Ferrara, F.; Lessi, F.; Vitagliano, O.; Birkenghi, E.; Rossi, G. (2019). Current Therapeutic Results and Treatment Options for Older Patients with Relapsed Acute Myeloid Leukemia. Cancers, 11, 224.
View at Publisher | View at Google Scholar - Hussein BA, Hallner A, Wennström L, Brune M, Martner A, Hellstrand K, et al. (2021) Impact of NK cell activating receptor gene variants on receptor expression and outcome of immunotherapy in acute myeloid leukemia. Front Immunol 12:796072.
View at Publisher | View at Google Scholar - Kaweme NM, Zhou F. (2021). Optimizing NK cell-based immunotherapy in myeloid Leukemia:Abrogating an immunosuppressive microenvironment. Front Immunol 12:683381.
View at Publisher | View at Google Scholar - Pizzolo G, Trentin L, Vinante F, Agostini C, Zambello R, Masciarelli M, et al. Natural killer cell function and lymphoid subpopulations in acute non-lymphoblastic leukemia in complete remission. Br J Cancer (1988) 58:368-372.
View at Publisher | View at Google Scholar - Rey J, Fauriat C, Kochbati E, Orlanducci F, Charbonnier A, D’Incan E, et al. (2017). Kinetics of cytotoxic lymphocyte reconstitution after induction chemotherapy in elderly AML patients reveals progressive recovery of normal phenotypic and functional features in NK cells. Front Immunol 8:64.
View at Publisher | View at Google Scholar - Dunbar EM, Buzzeo MP, Levine JB, Schold JD, Meier-Kriesche HU, Reddy V. (2008) The relationship between circulating natural killer cells after reduced-intensity conditioning hematopoietic stem cell transplantation and relapse-free survival and graft-versus-host disease. Haematologica 93(12):1852-1858.
View at Publisher | View at Google Scholar - Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci M-J, Reviron D, et al. Defective expression and function of natural killer cell–triggering receptors in patients with acute myeloid leukemia. Blood 2002;99(10):3661-3667.
View at Publisher | View at Google Scholar - De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, et al. (2003). The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30, and NKp44). Eur J Immunol;33(9):2410-2418.
View at Publisher | View at Google Scholar - Fauriat C, Just-Landi S, Mallet F, Arnoulet C, Sainty D, Olive D, et al. (2007). Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood;109(1):323-330.
View at Publisher | View at Google Scholar - Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R, et al. (2014). Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica;99(5):836-847.
View at Publisher | View at Google Scholar - Le Garff-Tavernier M, Decocq J, de Romeuf C, Parizot C, Dutertre CA, Chapiro E, et al. (2010). Analysis of Cd16+Cd56dim nk cells from cll patients: Evidence supporting a therapeutic strategy with optimized anti-Cd20 monoclonal antibodies. Leukemia 25(1):101-109.
View at Publisher | View at Google Scholar - Dreger P, Corradini P, Kimby E, Michallet M, Milligan D, Schetelig J, et al. (2007). Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: The ebmt transplant consensus. Leukemia 21(1):12-17.
View at Publisher | View at Google Scholar - Gribben JG. (2018). How and when I do allogeneic transplant in cll. Blood 132(1):31-39.
View at Publisher | View at Google Scholar - Cayssials E and Guilhot F: (2017). Chronic myeloid leukemia: Immunobiology and novel immunotherapeutic approaches. BioDrugs 31: 143-149.
View at Publisher | View at Google Scholar - Mumprecht S, Schürch C, Schwaller J, (2009). Solenthaler M and Ochsenbein AF: Programmed death 1 signaling on chronic myeloid leukemia‑specific T cells result in T‑cell exhaustion and disease progression. Blood 114: 1528‑1536.
View at Publisher | View at Google Scholar - Jørgensen HG, Lin A, Gaal K, (2016.). Holyoake TL and Bhatia R: Inhibition of interleukin‑1 signaling enhances elimination of tyrosine kinase inhibitor‑treated CML stem cells. Blood 128: 2671-2682,
View at Publisher | View at Google Scholar - Zhao K, Yuan S, Yin L, Xia J and Xu K: (2017). Potential efficacy of human IL‑1RAP specific CAR‑T cell in eliminating leukemic stem cells of chronic myeloid leukemia. J Leukemia 5: 232,
View at Publisher | View at Google Scholar - Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 99:754-758.
View at Publisher | View at Google Scholar - Miller JS, Soignier Y, PanoskaltsisMortari A, McNearney SA, Yun GH, Fautsch SK, et al. (2002). Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. x;105(8):3051-3057.
View at Publisher | View at Google Scholar - Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333-339
View at Publisher | View at Google Scholar - Locatelli F, Pende D, Mingari MC, Bertaina A, Falco M, Moretta A, et al. (2013). Cellular and molecular basis of haploidentical hematopoietic stem cell transplantation in the successful treatment of high-risk leukemias: Role of alloreactive NK cells. Frontiers in Immunology.;4:15.
View at Publisher | View at Google Scholar - Heidenreich S, Kroger N. (2017). Reduction of relapse after unrelated donor stem cell transplantation by KIRbased graft selection. Frontiers in Immunology.; 8:41.
View at Publisher | View at Google Scholar - Babor F, Manser AR, Fischer JC, Scherenschlich N, Enczmann J, Chazara O, Moffett A, Borkhardt A, Meisel R, Uhrberg M. KIR ligand C2 is associated with increased susceptibility to childhood ALL and confers an elevated risk for late relapse. Blood. 2014 Oct 2;124(14):2248-2251.
View at Publisher | View at Google Scholar - Ljunggren H-G, Kärre K. (1990). In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today.; 11:237-244.
View at Publisher | View at Google Scholar - Zhou Z, Zhang C, Zhang J, Tian Z. (2012). Macrophages help NK cells to attack tumor cells by stimulatory NKG2D ligand but protect themselves from NK killing by inhibitory ligand Qa-1. PLoS ONE.;7(5): 36928.
View at Publisher | View at Google Scholar - Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F. (2002). Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science.;295(5562):2097-2100.
View at Publisher | View at Google Scholar - Ruggeri L, Mancusi A, Perruccio K, Burchielli E, Martelli MF, Velardi A. Natural killer cell alloreactivity for leukemia therapy. J Immunother. 2005;28(3):175-182.
View at Publisher | View at Google Scholar - Chan YLT, Zuo J, Inman C, Croft W, Begum J, Croudace J, Kinsella F, Maggs L, Nagra S, Nunnick J. (2018). NK cells produce high levels of IL-10 early after allogeneic stem cell transplantation and suppress development of acute GVHD. Eur J Immunol.;48(2):316-329.
View at Publisher | View at Google Scholar - Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, Marsh SG, Geraghty D, Spellman S, Haagenson MD, Ladner M, Trachtenberg E, Parham P, Miller JS. (2010). Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood.;116(14):2411-2419.
View at Publisher | View at Google Scholar - Pittari G, Fregni G, Roguet L, Garcia A, Vataire A, Wittnebel S, Amsellem S, Chouaib S, Bourhis J, Caignard A. (2010). Early evaluation of natural killer activity in post-transplant acute myeloid leukemia patients. Bone Mar row Transplant.;45(5):862–871.
View at Publisher | View at Google Scholar - Cichocki F, Cooley S, Davis Z, DeFor TE, Schlums H, Zhang B, Brunstein CG, Blazar BR, Wagner J, Diamond DJ. (2016). CD56 dim CD57+ NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia.;30(2):456-463.
View at Publisher | View at Google Scholar - Zeidner JF, Foster MC. (2015). Immunomodulatory drugs: IMiDs in acute myeloid leukemia (AML). Curr Drug Targets.
View at Publisher | View at Google Scholar - Berg SL, Cairo MS, Russell H, Ayello J, Ingle AM, Lau H, et al. (2011). Safety, pharmacokinetics, and immunomodulatory effects of lenalidomide in children and adolescents with relapsed/refractory solid tumors or myelodysplastic syndrome: a Children’s Oncology Group Phase I Consortium report. J Clin Oncol 29(3):316-323.
View at Publisher | View at Google Scholar - Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. (2001) Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 98(1):210-216.
View at Publisher | View at Google Scholar - (138) . Bartlett JB, Michael A, Clarke IA, Dredge K, Nicholson S, Kristeleit H, et al. (2004). Phase I study to determine the safety, tolerability and immunostimulatory activity of thalidomide analogue CC-5013 in patients with metastatic malignant melanoma and other advanced cancers. Br J Cancer 90(5):955-961.
View at Publisher | View at Google Scholar - Xu J, Niu T. (2020). Natural killer cell-based immunotherapy for acute myeloid leukemia. J HematolOncol. Dec 7;13(1):167.
View at Publisher | View at Google Scholar - Cummins KD, Gill S. Chimeric antigen receptor T-cell therapy for acute myeloid leukemia: how close to reality? Haematologica. 2019;104(7):1302-1308.
View at Publisher | View at Google Scholar - Klingemann H. (2014). Are natural killer cells superior CAR drivers? Oncoimmunology.;3:28147.
View at Publisher | View at Google Scholar - Hauswirth AW, Florian S, Printz D, Sotlar K, Krauth MT, Fritsch G, Schernthaner GH, Wacheck V, Selzer E, Sperr WR. (2007). Expression of the target receptor CD33 in CD34+/CD38−/CD123+ AML stem cells. Eur J Clin Investing.;37(1):73-82.
View at Publisher | View at Google Scholar

 Clinic
Clinic
