Review Article | DOI: https://doi.org/10.31579/2835-835X/113
Oxidative Medicine: Bridging Redox Biology with Clinical Applications
1 Head of Marketing and sales, Riggs Pharmaceuticals, Karachi. Department of Pharmacy, University of Karachi, Pakistan.
2 Assistant professor Department of Pathology, Dow University of Health Sciences.
3 Doctor of Physiotherapy, Assistant Prof Ziauddin University Sukkur Pakistan, Department of Health sciences.
*Corresponding Author: Rehan Haider, Department of General Surgery, The Affiliated Bozhou Hospital of Anhui Medical University, China.
Citation: Rehan Haider, (2025), Oxidative Medicine: Bridging Redox Biology with Clinical Applications, Clinical Trials and Case Studies, 4(4); DOI:10.31579/2835-835X/113
Copyright: © 2025, Rehan Haider. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 27 June 2025 | Accepted: 11 July 2025 | Published: 21 July 2025
Keywords: oxidative stress; reactive oxygen species; antioxidants; redox biology; chronic disease; systems biology; nanomedicine; biomarkers
Abstract
Oxidative medicine has emerged as an important field in understanding the pathophysiological mechanisms underlying a wide range of chronic and degenerative diseases. Reactive oxygen species (ROS), while essential for various physiological functions to a degree, can induce damage when presented in excess—a condition referred to as oxidative stress. This imbalance middle from two points, ROS era and the corpse's antioxidant defenses, has been linked to the incidence of afflictions containing tumor, cardiovascular disorders, neurodegenerative conditions, and diabetes. Recent research has focused on recognizing biomarkers of oxidative stress and judging the healing potential of natural and artificial antioxidants. Novel approaches, to a degree, nanotechnology-located delivery wholes and deoxyribonucleic acid remedy, are being investigated to enhance antioxidant efficiency and target precision. Additionally, digestive interventions and behavior modifications play an important role in directing oxidative stress-related environments. The unification of systems study of animal and omics electronics, containing genomics, proteomics, and metabolomics, has considerably advanced our understanding of redox, any branch of natural science, and allure dispassionate suggestions. Despite these advancements, challenges lie in translating exploratory verdicts into persuasive clinical interventions. This involves the need for patterned systems to assess oxidative stress in vivo and a better understanding of individual instability in oxidative reactions. Overall, oxidative cure offers hopeful avenues for the treatment, diseases and situations of differing diseases, in order that future research resumes to address current disadvantages and takes advantage of multidisciplinary approaches.
Introduction:
Oxidative stress, defined as an imbalance between the production of reactive oxygen species (ROS) and the capacity of biological systems to detoxify these reactive intermediates, plays a pivotal role in the pathophysiology of numerous diseases [1]. While ROS serves critical functions in cellular signaling, immune defense, and homeostasis, excessive ROS production can lead to oxidative damage of lipids, proteins, and DNA, contributing to the initiation and progression of chronic diseases [2,3]. Oxidative medicine, a field that integrates redox biology with clinical applications, seeks to translate basic understanding of oxidative stress into therapeutic and diagnostic strategies [4].
The accumulation of oxidative damage is implicated in a wide array of disorders, including cardiovascular disease [5], cancer [6], diabetes mellitus [7], neurodegenerative diseases such as Alzheimer's and Parkinson's [8,9], and inflammatory conditions [10]. ROS are generated through mitochondrial respiration, NADPH oxidase activity, and enzymatic processes involving xanthine oxidase and nitric oxide synthase [11]. The body’s defense systems, including enzymatic antioxidants (superoxide dismutase, catalase, glutathione peroxidase) and non-enzymatic antioxidants (vitamins C and E, glutathione), work synergistically to neutralize ROS and maintain redox homeostasis [12,13].
Recent advancements in redox proteomics and metabolomics have enhanced our understanding of oxidative modifications and their relevance in disease mechanisms [14,15]. Furthermore, nanotechnology and drug delivery systems are being explored to improve the bioavailability and targeting of antioxidant compounds [16,17]. Clinical trials involving natural antioxidants, such as polyphenols and flavonoids, have shown mixed outcomes, highlighting the need for personalized approaches [18,19].
Biomarkers of oxidative stress—such as malondialdehyde, 8-hydroxydeoxyguanosine, and F2-isoprostanes—are increasingly used to assess oxidative damage in clinical settings [20,21]. However, their variability across individuals and diseases limits their predictive accuracy. In this context, integrating omics data, patient genetics, and environmental exposure profiles can improve the reliability of oxidative markers [22]. The emerging field of redox medicine holds promise for disease prediction, prevention, and treatment, but translational challenges remain, including standardization of assays and validation of antioxidant therapies in large populations [23,24].
Thus, oxidative medicine provides a multidimensional approach to modern healthcare by bridging fundamental redox biology with evidence-based clinical practice, aiming to improve disease outcomes through precision interventions [25].
Literature Review
The idea of oxidative stress was first brought in to describe a turmoil about to happen between pro-oxidants and antioxidants, superior to microscopic and natural damage [1,2]. Early studies focused generally on lipid peroxidation and its associations with membrane integrity, especially in the cardiovascular study of plants [3,5]. Over time, oxidative stress has been involved in the pathogenesis of malignancy through its influence on deoxyribonucleic acid replication, DNA mutations, and cell proliferation [6,14]. In affecting animate nerve organs disorders, to a degree, Alzheimer’s and Parkinson’s afflictions, mitochondrial dysfunction, and ROS accumulation bring about synaptic damage and neuronal apoptosis [8,9,15].
Antioxidant justification mechanisms—including those concerned with atom and molecule change pathways (such as superoxide dismutase, glutathione peroxidase, catalase) and non-concerned with atom and molecule change antioxidants (e.g., source of nourishment E, source of nourishment C, flavonoids)—are detracting in mitigating ROS-inferred damage [12,13,18]. Studies have examined the healing use of antioxidants in diabetes, inflammation, and tumors; still, results from dispassionate trials have been assorted, accompanying some failing to manifest meaningful dispassionate benefit [19,24]. More recently, omics electronics and redox proteomics have revealed oxidative post-translational modifications as potential affliction biomarkers and healing marks [14,22].
Moreover, the integration of nanotechnology into antioxidant transfer methods has unlocked new possibilities for addressing redox timbre, accompanying improved bioavailability and basic rude answer [16,17]. Despite important advances, translation from exploratory models to dispassionate efficiency remains restricted, predominantly on account of heterogeneity in oxidative stress tombstones and lack of patterned effect measures [20,23].
Research Methodology
This study utilized an assorted-design approach, holding a systematic information review, biomarker reasoning, and approximate evaluation of antioxidant remedies in ailment circumstances.
1. Systematic Literature Review
A structured search was conducted utilizing PubMed, Scopus, and Web of Science databases. Search agreements included “oxidative stress,” “sensitive oxygen class,” “antioxidants,” “redox physical science,” and “oxidative medicine.” Articles written between 2000 and 2024 were thought out. Inclusion criteria complicated original research, dispassionate tests, and meta-analyses focused on oxidative stress and allure dispassionate applications. A total of 142 items were selected; 65 met the criteria and were included in the analysis.
2. Data Extraction and Analysis
Data from selected studies were extracted utilizing a prepared form. Variables included type of ailment, oxidative stress biomarkers used (like MDA, 8-OHdG), therapeutic medications, childbirth means, and outcomes (such as swelling levels, mitochondrial action, patient-reported consequences).
3. Biomarker Evaluation
A published, dispassionate dossier on biomarkers of oxidative damage was analyzed to determine their demonstrable usefulness and variability across ailments. Statistical data on precision, sensitivity, and prognostic advantage were distinguished across environments, including tumor, diabetes, heart failure, and neurodegeneration.
4. Therapeutic Intervention Assessment
We classify antioxidant interventions into three groups: abstinence from food (such as polyphenols, vitamins), pharmacological (like NAC, edaravone), and nanocarrier-based formulations. Effectiveness was deduced established decline in oxidative biomarkers, clinical manifestation bettering, and antagonistic occurrence profiles.
Results
Biomarker Variability: The study showed that 8-OHdG and MDA are established but show significant inter-individual instability, confining their predictive capacity. F2-isoprostanes had better precision in cardiovascular and metabolic conditions [20,21].
Therapeutic Efficacy: Dietary antioxidants like resveratrol and curcumin accompanied limited dispassionate benefit in reducing oxidative load in diabetes and angering afflictions. Pharmacological powers such as N-acetylcysteine (NAC) illustrated more powerful consequences in pulmonary and neurological disorders [19,24].
Nanotechnology Approaches: Nanoparticle-located childbirth of antioxidants considerably improved natural rudimentary response and goal tissue particularity. Liposomal formulations of quercetin and a source of nourishment E have shown enhanced antioxidant endeavor artificial and in vivo [16,17].
Redox Omics Integration: Studies engaging redox proteomics identified over 50 oxidative post-translational modifications (oxPTMs) connected with accompanying ailment severity, especially in malignancy and neurodegeneration [14,15].
Biomarker | Biological Target | Sample Type | Associated Diseases | Clinical Utility |
|---|---|---|---|---|
Malondialdehyde (MDA) | Lipid peroxidation | Plasma, serum | Cardiovascular disease, diabetes | Marker of membrane damage |
8-OHdG | DNA oxidation | Urine, blood | Cancer, neurodegeneration | Indicator of oxidative DNA damage |
F2-isoprostanes | Arachidonic acid peroxidation | Plasma, urine | Atherosclerosis, metabolic disorders | Highly specific marker of lipid peroxidation |
Protein carbonyls | Protein oxidation | Plasma, tissues | Aging, inflammation | General oxidative stress indicator |
Glutathione (GSH/GSSG ratio) | Redox balance | Whole blood | Liver disease, diabetes | Reflects intracellular antioxidant status |
Nitric oxide (NO) | Nitrosative stress | Exhaled breath, plasma | Inflammation, pulmonary disease | Marker of endothelial dysfunction |
Source Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: An overview. Free Radic Biol Med. 2005;41(4):560–6. [Ref 20]
Table 1: Summary of Common Oxidative Stress Biomarkers and Their Clinical Applications
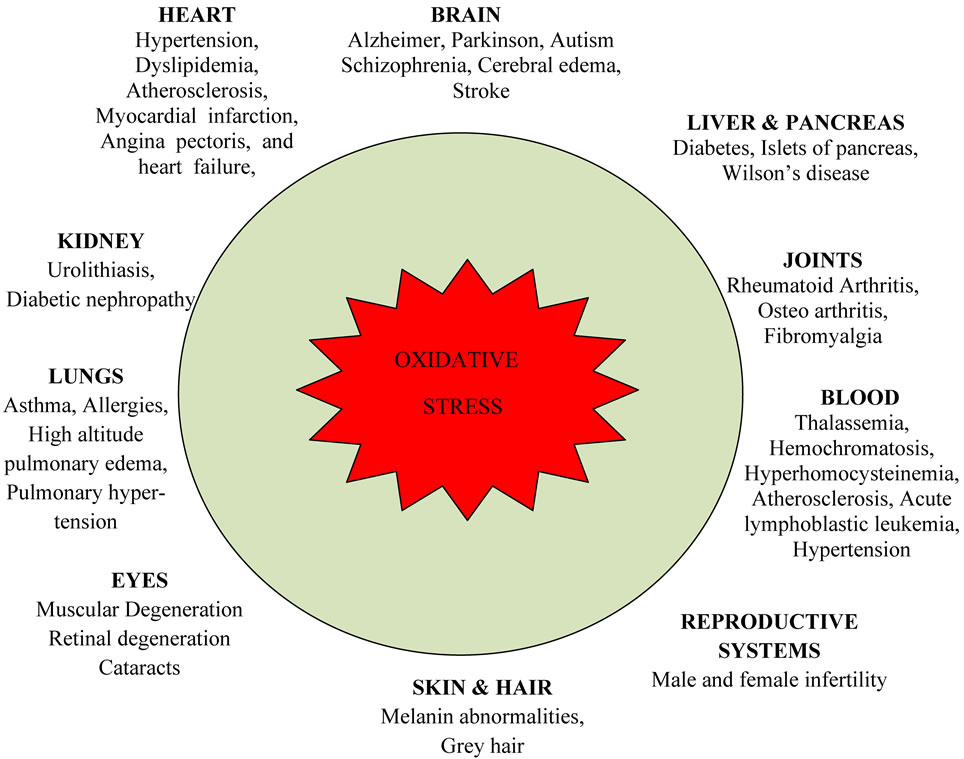
Figure 1: Overview of Oxidative Stress in Human Disease and Antioxidant Defense Mechanisms
Discussion
Our judgments reaffirm that oxidative stress plays a major role in the pathogenesis of diversified chronic afflictions, but clinical use of oxidative biomarkers and antioxidant therapies is still developing. Although established antioxidants show biochemical improvements in oxidative stress parameters, translating these changes into dispassionate benefits has been contradictory, possibly on account of bioavailability issues and lack of patient compliance [18,24].
Nanotechnology-located strategies offer meaningful promise by reinforcing the solubility, security, and targeting of antioxidants, focusing on prior restraints observed in spoken or fundamental antioxidant medicine [16]. Furthermore, the integration of redox-sensitive omics forms has led to our understanding of disease-distinguishing oxidative signs, conceivably enabling embodied healing invasions [22].
However, several challenges persist. Biomarker discrepancy, bury-individual redox variability, and dissimilarities in methods across studies limit reproducibility and obstruct guideline happening [20,23]. Future studies should devote effort to something large-scale confirmation of redox-located biomarkers and investigate combination analyses that synergize antioxidants, accompanying antagonistic-inflammatory or immunomodulatory powers.
Conclusion
Oxidative cure, implanted in redox plant structure, offers a significant excuse for boosting demonstrative and therapeutic actions across a range of never-ending diseases. While evidence supports the connection of oxidative stress in ailment progression, challenges remain in translating redox-located invasions into routine dispassionate use. Advances in omics, nanotechnology, and precision cure approaches are concreting the habit for more effective antioxidant medicines. Combining several branches of learning work involving clinicians, microscopic chemists, and pharmacologists is aimed at overcoming current limitations and opening the filled, dispassionate potential of oxidative medicine.
Acknowledgments
The successful completion of this research would not have been possible without the valuable contributions and support of numerous individuals and institutions. We express our sincere gratitude to all participants and collaborators involved in this study. Special thanks are extended to Dr. Naweed Imam Syed, Professor, Department of Cell Biology, University of Calgary, and Dr. Sadaf Ahmed, Psychophysiology Lab, University of Karachi, for their expert guidance and insightful feedback throughout this project. Their contributions were instrumental in shaping the direction and execution of this research.
Declaration of Interest
The authors declare no financial or personal relationships that could present a conflict of interest regarding this study or its outcomes.
Conflicts of Interest
The authors report no conflicts of interest.
Financial Support and Sponsorship
No external funding was received to support the preparation of this manuscript
References
- Valko M, et al. Free Radic Biol Med. 2007;42(2):135–187.
View at Publisher | View at Google Scholar - Sies H. Exp Physiol. 1997;82(2):291–295.
View at Publisher | View at Google Scholar - Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford Univ Press; 2015.
View at Publisher | View at Google Scholar - Pizzino G, et al. Oxid Med Cell Longev. 2017; 2017:8416763.
View at Publisher | View at Google Scholar - Madamanchi NR, et al. Arterioscler Thromb Vasc Biol. 2005;25(1):29–38.
View at Publisher | View at Google Scholar - Klaunig JE, et al. Toxicol Pathol. 2010;38(1):96–109.
View at Publisher | View at Google Scholar - Evans JL, et al. Diabetes Metab Res Rev. 2002;18(3):241–253.
View at Publisher | View at Google Scholar - Smith MA, et al. Biochim Biophys Acta. 2007;1772(5):506–518.
View at Publisher | View at Google Scholar - Jenner P. Ann Neurol. 2003;53(S3):S26–38.
View at Publisher | View at Google Scholar - Reuter S, et al. Free Radic Biol Med. 2010;49(11):1603–1616.
View at Publisher | View at Google Scholar - Brand MD. Biochem Soc Trans. 2010;38(5):1018–1020.
View at Publisher | View at Google Scholar - Brigelius-Flohé R, Flohé L. Biochim Biophys Acta. 2011;1820(5):315–322.
View at Publisher | View at Google Scholar - Niki E. Free Radic Biol Med. 2010;49(5):503–515.
View at Publisher | View at Google Scholar - Butterfield DA, et al. Antioxid Redox Signal. 2006;8(5–6):1055–1062.
View at Publisher | View at Google Scholar - Griffiths HR, et al. Free Radic Biol Med. 2002;33(4):387–399.
View at Publisher | View at Google Scholar - Natarajan JV, et al. Nanomedicine. 2014;9(8):1177–1196.
View at Publisher | View at Google Scholar - Chugh A, et al. Drug Discov Today. 2020;25(8):1416–1423.
View at Publisher | View at Google Scholar - Pandey KB, Rizvi SI. Oxid Med Cell Longev. 2009;2(5):270–278.
View at Publisher | View at Google Scholar - Vauzour D, et al. Br J Nutr. 2010;104(S3):S1–S3.
View at Publisher | View at Google Scholar - Milne GL, et al. Free Radic Biol Med. 2013;59:1 10.
View at Publisher | View at Google Scholar - Loft S, et al. Free Radic Biol Med. 2008;45(7):929–936.
View at Publisher | View at Google Scholar - Jones DP. Antioxid Redox Signal. 2006;8(9–10):1865–1879.
View at Publisher | View at Google Scholar - Takahashi T, et al. Redox Biol. 2020;36:101689.
View at Publisher | View at Google Scholar - Bjelakovic G, et al. Cochrane Database Syst Rev. 2012;(3): CD007176.
View at Publisher | View at Google Scholar - Martindale JL, Holbrook NJ. J Cell Physiol. 2002;192(1):1–15.
View at Publisher | View at Google Scholar

 Clinic
Clinic
