Review Article | DOI: https://doi.org/10.31579/2834-5126/010
Osteoporosis Management: A Suggested Algorithm for Optimizing Spine Surgery Outcomes in Osteoporotic Patients
1 University of Utah School of Medicine, Salt Lake City, Utah, USA.
2 Institute of Medical Sciences and SUM hospital, Bhubaneswar, India.
3Albany Medical College, Albany, New York, USA.
4 Department of Neurosurgery, University of Florida, Gainesville, Florida, USA.
*Corresponding Author: Brandon Luck-Wold, Department of Neurosurgery, University of Florida, Gainesville, Florida, USA.
Citation: Brandon Luck-Wold, Matthew C. Findlay, Kyril L. Cole, Mrinmoy Kundu (2023), Osteoporosis Management: A Suggested Algorithm for Optimizing Spine Surgery Outcomes in Osteoporotic Patients. Clinical Trials and Clinical Research. 2(1); DOI:10.31579/2834-5126/010
Copyright: © 2023 Brandon Luck-Wold, this is an open access article distributed under the creative commons’ attribution license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 02 November 2022 | Accepted: 16 December 2022 | Published: 20 January 2023
Keywords: osteoporosis; optimized management; improved algorithm, post-operative recovery
Abstract
As a common metabolic bone disease that affects millions, osteoporosis has been classically considered a contraindication to spine surgery. However, despite the higher risk of operating on an osteoporotic spine, surgeons have found that through preoperative preparation and adapted intraoperative and postoperative management strategies, patients can experience significantly improved odds of a successful surgery. No broad-scale algorithm has yet been proposed which outlines the diagnostic, preoperative, intraoperative, and postoperative interventions which are validated to optimize osteoporosis patient outcomes. In the present article, we suggest such an algorithm and otherwise discuss the natural history of osteoporosis, the process of the diagnostic workup, the perioperative multidisciplinary approach to osteoporosis care, and the intraoperative and postoperative strategies which allow the best spine surgery outcomes in the setting of osteoporosis.
Introduction
As life expectancies continue to rise globally, increasing numbers of elderly individuals seek surgical interventions to rectify degenerative conditions and ameliorate chronic pain. This is evidenced by the ever-growing rates of spine surgery. For example, from 2004-2015, the number of spinal fusions performed in the United States increased by 276%. [1] Unfortunately, many who would greatly benefit from spinal surgery are hindered by osteoporosis, a classic contraindication to spine surgery. As the most common metabolic bone disease, osteoporosis afflicts over 10.3 million older US adults (>50 years) and portends higher rates of fracture, progressive spinal deformities, and stenosis following spine surgery.[2,3] Concerning increasing life expectancies, the quantity of patients with osteoporosis is steadily, as is the frequency with which osteoporosis impacts spinal care.[4] Despite the higher risk of operating on an osteoporotic spine, surgeons have found that careful patient selection, deliberate preoperative preparation, modified surgery techniques, and active postoperative management can provide good surgical outcomes with acceptable risk rates [5]. In particular, data show that preoperative treatment of suboptimal bone health, like in osteoporosis, leads to higher rates of successful surgical outcomes [6,7].
Recently, the first-ever guidelines for assessing and managing osteoporosis in patients undergoing elective spinal reconstruction were published [8]. These guidelines identify preoperative bone health quantification tools and suggest limited presurgical treatment protocols. However, no broad-scale algorithm has been proposed that describes the diagnostic, preoperative, intraoperative, and postoperative management strategies that optimize osteoporosis spine surgery outcomes [8]. In the present article, we suggest such an algorithm and otherwise discuss the natural history of osteoporosis, the process of the diagnostic workup, the perioperative multidisciplinary approach to osteoporosis care, and the intraoperative and postoperative strategies which portend improved spine surgery outcomes in the setting of osteoporosis.
Natural History
Pathogenesis
Osteoporosis is a disease characterized by a significant increase in bone fragility consequential by excessive osteoclastic bone resorption and insufficient osteoblastic formation.9 This function disequilibrium precipitates defects in trabecular microarchitecture and an impaired capacity to repair microdamage from normal activities of daily living [10]. Osteoporosis is known to compromise the integrity of both trabecular bone and cortical bone, with a loss of trabecular integrity bearing greater relation to fractures of the spine and hips [11]. Studies indicate that a 10% loss of bone mass effectively doubles the risk of vertebral fracture.12 While hip and spine fractures have proven most problematic, the systemic increases in bone fragility associated with advanced osteoporosis are such that nearly any bone can fracture [12]. Directly implicated in the onset of osteoporosis are deficiencies of calcium and estrogen [13]. Indirectly implicated are lifestyle factors, such as smoking, alcohol consumption, and inadequate exercise [14]. Other modifiable and nonmodifiable risk factors exist for osteoporosis and are listed in Table 1 [15].
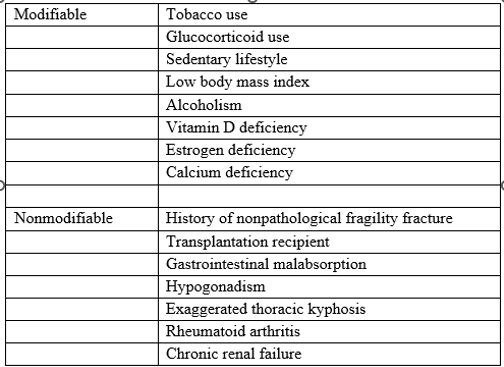
Table 1: Osteoporosis risk factors
Table adapted from Fiani et al [15].
Prevalence and Cost
Women face the greatest risk of osteoporosis. Approximately one in three women >50 and one in five men >50 are at risk of osteoporosis [16]. Estrogen deficiency is largely responsible for osteoporosis in postmenopausal women [17]. The medical costs of osteoporosis in the USA during 2008 were estimated to exceed 22 billion dollars [18]. According to a report by the US Surgeon General [19], more than 10 million Americans >50 have osteoporosis, with an additional 34 million at high risk for the disease [20].
Outcomes, Lifestyle, Morbidity
Osteoporosis is currently one of the leading causes of morbidity and mortality worldwide [21]. It is estimated that up to 20% of patients die within the first year following hip fractures related to osteoporosis [22]. Moreover, those who experience fractures of the spine or hip due to osteoporosis often face significant lifestyle restrictions thereafter. Hip fractures are regarded as the most severe complications, frequently causing loss of self-sufficiency and permanent physical disability [21]. It is estimated that less than half of those who survive hip fractures regain previous levels of physical function [22]. Approximately 50% of older women with osteoporosis who experience a hip fracture will lose the ability to walk again [23].
Prevention
Calcium and vitamin D are both essential to the process of bone formation. Thus, as public health measures, supplementing these elements is standardly recommended for prophylaxis and treatment of osteoporosis [16]. Physical activity is another direct contributor to bone health, with weight-bearing exercise most prominently increasing bone density [24]. For young females especially, adequate calcium intake and sufficient weight-bearing physical activities are advised as highly effective prophylactic measures to stave off osteoporosis in their later years [25].
Diagnostic Workup for Osteoporosis
Common Presentations: Signs and Symptoms
Osteoporosis is often referred to as a silent disease, as those affected are generally asymptomatic for years to decades before any sign or clinical detection [26]. As a result, those affected are often diagnosed only after their first pathologic fracture [27]. Several risk factors exist that put individuals at an increased likelihood of complications related to osteoporosis. For spine surgeons and all physicians alike, typical important risk factors queried include advanced age, female sex, Caucasian race, sedentary lifestyle, family history of multiple fractures or osteoporosis, small body frame or low body weight, postmenopausal status, and a history of smoking [28,29].
When evaluating a patient for possible osteoporosis, it is important to note that there often may be no signs or symptoms at the time of clinical assessment, as the first clinical manifestation often presents in advanced stages with pathologic fractures [27]. However, among individuals with symptomatic osteoporosis, several symptoms have been commonly described, often detailing no history of preceding trauma or known triggers for their symptoms. These may include new or chronic bone pain, joint pain, back pain, or fractures of bones occurring without trauma [30]. New functional impairments, seen as difficulty bending, lifting, walking, or climbing, may also be described [31]. Regarding the involvement of the spine, clinical manifestations of symptomatic vertebral fractures can include a noticeable loss in height, a noted stooped posture resulting in a hunchback appearance, or descriptions of new neck or lower back pain failing to resolve [32]. An important note for advanced osteoporosis on patient history is that the acute onset of bone pain or fractures can occur from exceedingly minor movements. These fractures can occur spontaneously from minor events such as coughing or going over speed bumps in a vehicle [33].
As the symptoms of osteoporosis may vary, signs of the disease may also vary on evaluation. Upon patient examination, palpation of the affected bone(s) or joint(s) may result in point-specific pain and bony crepitus, with fractures frequently occurring in the hip, distal radius, proximal humerus, and spine [30]. Regarding spine care, osteoporotic vertebral compression fractures may be clinically assessed by measurement of height loss from baseline and physical findings indicating kyphosis of the spine [30]. A height loss of >6 cm has shown a specificity of >90% in detecting vertebral fractures [34]. However, this height loss may be insidious, having occurred over decades while remaining asymptomatic [32]. Physical findings of kyphosis are important, as they likely indicate multiple vertebral compression fractures [35]. It has been noted that each complete compression fracture can cause approximately 1 cm or more in diminished height, with a height loss >4 cm resulting in 15 degrees of kyphosis.36 While these positive signs and symptoms may help consider osteoporosis, variability in patient presentations can make it difficult to assess; thus, proper quantitative diagnostic testing is required for confirmation.
Diagnostic Testing
A complete history and physical exam are neither sensitive nor sufficient to diagnose primary osteoporosis properly [37] Plain radiographs and bone density testing should be pursued in patients with suspected osteoporosis to confirm the diagnosis [38]. Once findings consistent with osteoporosis are established, testing for the secondary cause of the osteoporosis can be pursued for the most effective disease management. An in-depth osteoporosis diagnostic workflow is depicted in Figure 1.
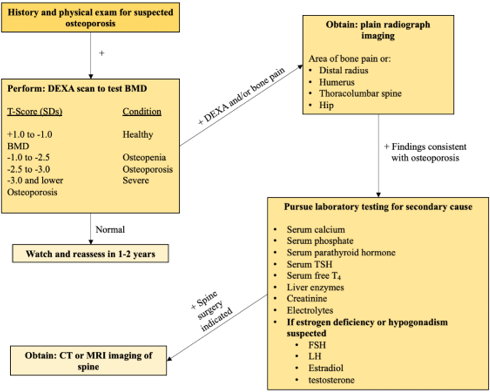
Figure 1: An in-depth diagnostic workflow to radiographically evaluate for the presence of osteoporosis. Once radiographic imaging is consistent with osteoporosis, laboratory studies are advised to characterize the underlying etiology of the low bone density and to inform medical therapies moving forward.
Because osteoporosis is characterized by low bone mass, microarchitectural disruptions, and increased skeletal fragility, bone mineral density (BMD) assessment by dual-energy x-ray absorptiometry (DEXA) has become the standard test for diagnosing osteoporosis [39]. DEXA scans allow for a quantitative assessment of an individual’s bone density and future fracture risk. With a DEXA scan complete, a T-score, or the standard deviation (SD) by which an individual’s BMD differs from the mean value expected in young healthy individuals, is then calculated for the patient’s bone density to be adequately assessed for osteoporosis. The World Health Organization defines osteoporosis as a T-score of < -2.5 SD, with normal bone density > -1.0 SD [40].
Concerning osteoporosis of the spine, plain radiographs of the thoracolumbar spine are paramount to confirm the location, type of vertebral abnormalities or fractures, and degree of severity of the bone disease [30,41]. The severity of vertebral fractures resulting from osteoporosis may be staged for further management planning [42]. Additional imaging, such as MRI or CT scans, may be considered if further diagnostic information is indicated, such as in determining the potential instability of a wedge fracture or for surgical versus non-surgical management planning [5,43]. More specifically, CT scans can best define the type of fracture, the extent of vertebral destruction, and the extent of spinal compression; MRI allows for the best assessment of the spinal cord and nerve rootlets for compressive damage while helping the spine surgeon differentiate between new and old fractures [30].
Osteoporosis versus Osteopenia in Spine Surgery
Osteopenia is a condition of “low bone mass,” lower than usual for an individual’s age [44]. The World Health Organization classifies osteopenia as a T-score between -1.0 and -2.5 SD. This is differentiated from osteoporosis, a more severe version of bone loss with a higher likelihood of fracturing and a T-score of < -2.5 SD [44]. Of note, treating your patient as having osteoporosis rather than osteopenia is recommended in patients with a T-score of -1.0 to -2.5 and a fragility fracture of the pelvis, wrist, or shoulder [45]. Regarding the epidemiology of the two bone diseases in spine surgery, there appears to be a discrepancy between males and females. Chin et al. found that among patients over [50]. who needed spine surgery, 46.1% of males and 41.4% of females demonstrated osteopenia, while 14.5% of males and 51.3% of females had osteoporosis on workup [46]. The prevalence of lower bone density in women, as seen in osteoporosis, is understandable given the deleterious effects of estrogen depletion post-menopause.
Limited data exist on the effects of decreased bone density and outcomes after spine surgery. However, in one study among adult patients undergoing single-level lumbar fusion, Khalid et al. found the odds of pseudoarthrosis and revision surgery to be significantly higher in those with osteopenia and osteoporosis than in patients with normal BMD [47]. As expected, patients with osteoporosis had higher odds of pseudoarthrosis and revision surgery (OR 1.92 and OR 3.25, respectively) than patients with osteopenia (OR 1.7 and OR 2.73, respectively) [47]. Whether a patient has osteopenia or osteoporosis; lower BMD can have deleterious effects on elective spine surgery, from screw pullout to poor fusion rates [30,48].
Multidisciplinary Management
Once a diagnosis of osteoporosis is made, a multidisciplinary team involving the spine surgeon, primary care physician, endocrinologist, and physical therapist is the ideal approach for preoperative medical optimization [4]. Each of these disciplines bring special skill sets. For example, spine surgeons must assess three factors in an osteoporotic patient. The first step involves properly diagnosing osteoporosis using a physical exam, quantitative imaging, and laboratory data [49]. The second step is to provide patients with medical care and lifestyle recommendations to increase BMD preoperatively [50,51]. The third step is managing any osteoporosis-related complications that arise as direct sequelae of the spine surgery [52].
Primary care physicians maintain a close relationship with the patients and maintain frequent patient contact throughout the treatment pipeline. Furthermore, primary care physicians are directly involved in evaluating preoperative risk vs. benefits, assisting in implementing lifestyle modifications, managing the outpatient aspects of postoperative complications, and helping organize long-term recovery.
Endocrinologists may be integral to treating any underlying causes precipitating the diminished bone density. Considering osteoporosis is often a secondary manifestation of conditions like hypo- or hyperthyroidism, hypogonadism, diabetes mellitus, growth hormone deficiency, or cancer, endocrinologists are critical in the diagnosis and management of these or any other additional pathologies impacting a patient’s candidacy for spine surgery [53].
Physical therapists serve to implement exercise-related therapies indicated for osteoporosis treatment and prevention [54]. These providers aid in pain management and guard against subsequent fracture development by improving patient exercise tolerance, mobility, flexibility, and strength [55].
Medical Therapies to Enhance Bone Density
Vitamin/mineral supplementation protocols or pharmacological regimens can enhance bone quality and significantly lower the risk of mechanical failure of spine constructions. Ideally, these therapies will be initiated in the preoperative period to prepare the bony spine for fusion [15]. Additionally, if possible, any intake of drugs that diminish bone density, such as glucocorticoids, should be stopped or reduced [15]. It is assumed that in emergent situations or in the presence of other contraindications, the necessity of surgery outweighs a prolonged time course for preoperative bone optimization, and thus bone-regenerative medical interventions may be started only postoperatively. Regardless of the initiation timeline, the medical optimization of patients’ bone health most commonly continues past the perioperative period into the long term.
Vitamin D and Calcium
Both calcium, a crucial building block of bone, and vitamin D, which increases calcium absorption, are often simple to sufficiently obtain from the diet. However, calcium and vitamin D supplementation is advised for elderly individuals who have a higher risk of bone loss. Animal models suggest promising surgical outcomes following calcium and vitamin D perioperative supplementation. In a study by Metzger et al., rats treated with a posterolateral spine fusion who had meals supplemented with vitamin D showed improved manual palpation fusion rates [56]. Particularly, decreased construct stiffness was consistently linked to vitamin D deficiency or insufficiency [56]. A similar improvement in mechanical strength and fusion volume was seen after calcium supplementation in ovariectomized rats that underwent posterolateral spine fusion. Their data showed that fusion masses in the calcium-supplemented group could endure 3-point bending forces that were 50% higher than the forces that the non-supplemented group could withstand[57].
Bisphosphonates
Bisphosphonates help inhibit bone resorption by inducing osteoclast apoptosis. Due to their strong affinity for bone and the phosphate and hydroxyl moieties they contain, bisphosphonates provide selective deposition throughout the whole skeleton. Although there is a plethora of studies on the subject, there is no universal agreement regarding the role of bisphosphonates in animal spine surgical models. Generally, however, the use of bisphosphonates has been linked to a higher percentages of immature bone in histologically examined fusion masses and randomized trials have shown biphosphate alendronate to demonstrate higher frequency of solid fusion and lower rates of cage subsidence [58,59]. While bisphosphonates show promise as a therapeutic approach, making specific recommendations on using bisphosphonates in the management of osteoporotic spine is currently challenging due to the dearth of relevant data.
Recombinant parathormone hormone (PTH)
Recombinant PTH is the only anabolic drug now offered to osteoporosis patients. Increased bone resorption and kidney calcium reabsorption are induced by endogenous PTH, which is necessary for raising serum calcium levels. According to animal models, rhPTH therapy results in higher fusion rates and better fusion structure [60-62]. Likewise, major randomized controlled trials have shown that recombinant human PTH (rhPTH) reduces fracture risk compared to placebo, and even more effectively than bisphosphonate therapy [63]. For example, in a clinical trial by Ohteri et al, posterolateral fusion patients with osteoporosis successfully fused at an 82% rate following rhPTH administration compared to a 68% rate following bisphosphonate administration. Additionally, literature shows the incidence of back pain is also lower in rhPTH-treated patients compared to placebo-treated patients, and patients who have the most severe baseline back pain may experience the most substantial decreases in pain symptoms, even after the rhPTH treatment is stopped [64].
Hormone replacement therapy
The treatment of osteoporosis in older adults also includes using estrogen and selective estrogen receptor modulators (SERMs). Compared to a placebo, these drugs reduce the risk of vertebral fractures by 50% while increasing bone mass [65]. Estrogen is no longer the first-line treatment for long-term osteoporosis management or fracture prevention due to its cardiovascular risk [66,67]. SERMs reduce spinal fractures by about 40% while increasing spinal fusion mass [68,69].
Calcitonin
Calcitonin, an intranasal osteoporosis drug, functions by directly suppressing osteoclasts. It has been demonstrated that 200 IU of Calcitonin reduces the risk of spinal fractures by 33% compared with a placebo [70]. Calcitonin is generally not the first-line for osteoporosis treatment, as it has shown limitations in preventing hip fractures in older patients. In animal studies, posterolateral fusion models in rabbits showed enhanced fusion rates and improved fusion histology [71]. while another study found that Calcitonin had a neutral effect[61].
Denosumab
Denosumab prevents RANKL from activating nuclear factor kappa-B ligand, preventing osteoclasts from resorbing bone. In clinical trials, denosumab was found to significantly reduce serum markers of bone turnover and increase bone mineral density in postmenopausal [72]. Orthopedic and spine surgeons are also interested in denosumab because its side effect profile is typically less severe than that of bisphosphonates.
Surgical Strategies
Once quantitative radiographic measures demonstrate adequate bone density, or when clinical judgment determines the cost-benefit risks of surgery are sufficiently favorable, osteoporosis patients will be recommended for spine surgery.
Bone mineral density directly impacts all biomechanical factors linked to spine surgery, including fatigue failure, pullout strength, and insertional torque [73]. Optimizing the bone-screw interface is essential for successful fixation because screw pullout or cutout is the most frequent cause of bone-implant failure in the osteoporotic spine [74]. Pedicle screw augmentation with calcium phosphate or polymethyl methacrylate (PMMA) to enhance fixing strength has been thoroughly studied [75-78]. The degree of the improvement in fixation is significantly influenced by the cement injection process and improvements in screw design. Fenestrated screws allow the cement to be contained to the vertebral body and prevent extrusion [75]. In contrast to cannulated or fenestrated screws, solid screws with retrograde cement prefilling appear to have higher pullout strength [76]. When PMMA is added, pullout strength is increased by 149%. Placing the screw through uncured PMMA rather than hardened cement produces better results [77]. Additionally, it has been noted that PMMA augmentation results in a higher fusion rate and less loss of deformity correction [78].
Additionally, recent advancements in pedicle screw design have improved fixation in osteoporotic bone. Expandable pedicle screws may increase pullout strength when treating traumatic and degenerative spinal disorders in people with osteoporosis [79]. Although the use of expandable pedicle screw systems has not been approved by the US FDA, one study found that expandable pedicle screws had a decreased risk of loosening or loss of fixation in lumbar spine fusion and may improve overall clinical outcomes [80]. Hybrid constructs (constructs that use hooks and wires) also may improve fixation secondary to the relative preservation of cortical bone in the lamina.
Although the American Academy of Orthopaedic Surgeons Clinical Practice Guidelines do not currently advocate vertebroplasty for the therapy of osteoporotic vertebral compression fracture, a growing body of research indicates that this procedure may have some application [81]. Theoretically, preventive vertebroplasty might lower the stiffness at the junction of the construct and the adjacent level, thus lowering the rate of junctional failure [82]. In order to stop future kyphosis next to lengthier segmental structures, vertebroplasty may also be helpful.
Postoperative Management
Immediate Postoperative Period
It can be inferred that the immediate postoperative care of osteoporotic patients will closely resemble that of non-osteoporotic cases. Early mobilization, adequate pain management, and recuperation from the physical trauma and anesthesia-related sequelae must be prioritized.
Regarding the immobilization of the healing spine, postoperative spinal bracing has been commonly used in non-osteoporotic post-surgical care to reduce patient pain, protect surgical sites, and enhance positive outcomes [83,84]. That said, amassing data report no evidence for pain reduction or heightened surgery success rates via bracing among non-osteoporotic patients [85-87]. Among osteoporotic patients, however, a 2019 systematic review which included four randomized controlled trials and three prospective cohort studies concluded that in elderly patients with osteoporotic compression vertebral fractures, spinal orthoses led to improved vertebral stability, reduced kyphotic deformity, enhanced postural stability, greater muscular strength and overall superior functional outcomes [88]. It is advisable, therefore, that osteoporotic patients receive postoperative bracing.
Long term Management
Multidisciplinary care teams are instrumental in effecting the continuation or initiation of the preoperative therapies described during the postoperative period. For osteoporotic patients who underwent emergent spine surgery and have yet to adequately diagnose or address the underlying etiologies or sequelae of their osteoporosis, a multidisciplinary team must be formed and management protocols initiated. Regardless of emergent or elective surgery timelines, endocrinologists will address any primary pathologies precipitating poor bone density, and physical therapists are paramount for increasing patients’ exercise tolerability and recovering musculoskeletal strength. Surgeons and primary care providers must follow up with patients to assess wound healing, symptom progression or alleviation, surgical construct fixation, and bony fusion formation. Medical therapies and vitamin supplementation must also be initiated or continued as some medical interventions, like bisphosphonates and rhPTH, may demonstrate their greatest effectiveness in promoting bone growth during the short-term postoperative period [89].
One postoperative intervention that may play a greater role in osteoporotic spinal care is external bone growth stimulators. These stimulators operate by pulsing electromagnetic fields to promote bone formation. Small animal models suggest these stimulators are effectively stimulate trabecular bone volume, though these results are less consistent in large animal models [90,91]. Likewise, data is inconclusive in human trials, and the overall strength of evidence advocating stimulator use is considered low [92]. Specific to osteoporosis, some studies have found stimulators to induce higher fusion rates in high-risk osteoporotic patients, while others have not [93]. Due to their high costs and controversial evidence, bone growth stimulators are not commonly utilized [90]. It remains to be seen whether bone stimulators will have a substantial role in the postoperative care of osteoporosis spine patients.
Perhaps the most important long-term management strategy involves lifestyle modifications. Optimized exercise habits, a nutrient-rich diet, avoidance of excessive alcohol consumption, smoking cessation, minimization of glucocorticoids, and mitigation of falls or other injuries will provide the best chance for long-term wound healing and solid bony fusion. Unfortunately, osteoporotic patients remain at high risk of fusion failure and carry 3-fold elevated odds of pseudarthrosis compared to those with adequate bone density [47]. For patients experiencing severe symptoms or progressive deformity, revision surgery may be necessary. In these cases, optimizing the preoperative, intraoperative, and postoperative treatment protocols is even more important to manage the patient’s low bone density to give the greatest chance of successful outcomes [94].
Proposed Algorithm
Considering the presently described preoperative, intraoperative, and postoperative protocols, which portend improved fusion outcomes in osteoporotic patients, we suggest the comprehensive care algorithm detailed in Figure 2. Dependent upon patient circumstances, presentation timeline, and resource availability, it may not be possible to implement all suggested diagnostic and therapeutic interventions. Flexibility and judgment are required in these cases to prioritize the most appropriate and efficacious therapies.
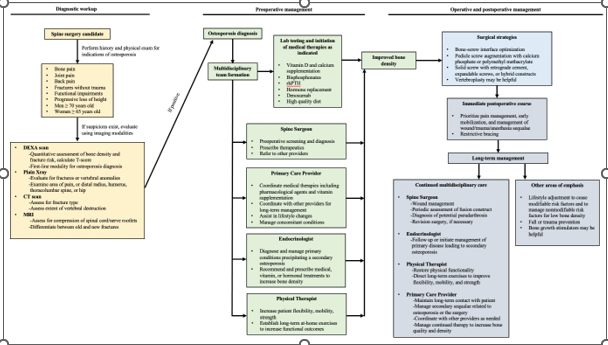
Figure 2 A comprehensive algorithm for the diagnosis, preoperative, intraoperative, and postoperative management of osteoporosis. Ideally patients would proceed from one end of the pipeline to the other. In cases of trauma or other idiosyncratic circumstances, surgical intervention may be required without adequate preoperative preparation. In these cases, postoperative management must perform the lab testing and subsequent initiation of any indicated medical therapies to provide the best chance for postoperative success.
Conclusions
Osteoporosis affects millions of Americans and many more worldwide. This low-bone density condition has important implications in spinal care and is strongly associated with poor surgical outcomes. As the number of patients with osteoporosis and concomitant spinal disease continues to rise, it is necessary to clearly define the preoperative, intraoperative, and postoperative treatment strategies which provide the greatest chance for long-term success. In the present work, we suggest a comprehensive care algorithm to guide clinicians in the care optimization for spine surgery patients with osteoporosis.
References
- Deng, H. Yue, J. K. Ordaz, A. Suen, C. G. & Sing, D. C. (2021). Elective lumbar fusion in the United States: national trends in inpatient complications and cost from 2002-2014. J Neurosurg Sci.503-512.
View at Publisher | View at Google Scholar - Tomé-Bermejo, F. Piñera, A. R. (2017). & Alvarez-Galovich, L. Osteoporosis and the Management of Spinal Degenerative Disease (I). Arch Bone Jt Surg. 272-282.
View at Publisher | View at Google Scholar - Wright, N. C. et al. (2014). The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2520-2526.
View at Publisher | View at Google Scholar - Mo, X. et al. (2021). High prevalence of osteoporosis in patients undergoing spine surgery in China. BMC Geriatrics. 361.
View at Publisher | View at Google Scholar - Lehman, R. A. J. Kang, D. G. & Wagner, S. C. (2015). Management of Osteoporosis in Spine Surgery. JAAOS - Journal of the American Academy of Orthopaedic Surgeons. 23:253-263.
View at Publisher | View at Google Scholar - Lubelski, D. Choma, T. J. Steinmetz, M. P. Harrop, J. S. & Mroz, T. E. (2015). Perioperative Medical Management of Spine Surgery Patients with Osteoporosis. Neurosurgery. 92-97.
View at Publisher | View at Google Scholar - Anderson, P. A. Kadri, A. Hare, K. J. & Binkley, N. Preoperative bone health assessment and optimization in spine surgery. Neurosurgical Focus.
View at Publisher | View at Google Scholar - Sardar, Z. M. et al. (2022). Best Practice Guidelines for Assessment and Management of Osteoporosis in Adult Patients Undergoing Elective Spinal Reconstruction.128-135.
View at Publisher | View at Google Scholar - Föger-Samwald, U. Dovjak, P. Azizi-Semrad, U. Kerschan-Schindl, K. & Pietschmann, P. (2020). Osteoporosis: Pathophysiology and therapeutic options. 1017-1037.
View at Publisher | View at Google Scholar - Armas, L. A. & Recker, R. R. (2012). Pathophysiology of osteoporosis: new mechanistic insights. 475-486.
View at Publisher | View at Google Scholar - Schaftenaar, W. (2020). The challenge of obtaining reference values for use in captive animals like elephants. 115-117.
View at Publisher | View at Google Scholar - Klotzbuecher, C. M. Ross, P. D. Landsman, P. B. Abbott, T. A. (2000). 3rd & Berger, M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. 721-739.
View at Publisher | View at Google Scholar - Prestwood, K. M. & Kenny, A. M. (1998). Osteoporosis: pathogenesis, diagnosis, and treatment in older adults. Clin Geriatr Med. 577-599.
View at Publisher | View at Google Scholar - Zhu, K. & Prince, R. L. (2015). Lifestyle and osteoporosis. Curr Osteoporos Rep. 52-59.
View at Publisher | View at Google Scholar - Fiani, B. et al. (2021). Special Considerations to Improve Clinical Outcomes in Patients with Osteoporosis Undergoing Spine Surgery. 386-401.
View at Publisher | View at Google Scholar - Li, H., Xiao, Z. Quarles, L. D. & Li, W. (2021). Osteoporosis: Mechanism, Molecular Target and Current Status on Drug Development. 1489-1507.
View at Publisher | View at Google Scholar - Management of osteoporosis in postmenopausal women: the 2021 position statement of The North American Menopause Society. 973-997.
View at Publisher | View at Google Scholar - Blume, S. W. & Curtis, J. R. (2011). Medical costs of osteoporosis in the elderly Medicare population. 1835-1844.
View at Publisher | View at Google Scholar - Reports of the Surgeon General. (2004). Bone Health and Osteoporosis: A Report of the Surgeon General.
View at Publisher | View at Google Scholar - Clynes, M. A. et al. (2020). The epidemiology of osteoporosis. 105-117.
View at Publisher | View at Google Scholar - Lamichhane, A. P. (2005). Osteoporosis-an update. JNMA J Nepal Med Assoc 44: 60-66 (2005).
View at Publisher | View at Google Scholar - Keene, G. S. Parker, M. J. & Pryor, G. A. (1993). Mortality and morbidity after hip fractures. 307:1248-1250.
View at Publisher | View at Google Scholar - Nazrun, A. S. Tzar, M. N. Mokhtar, S. A. & Mohamed, I. N. (2014). A systematic review of the outcomes of osteoporotic fracture patients after hospital discharge: morbidity, subsequent fractures, and mortality. 937-948.
View at Publisher | View at Google Scholar - Jendrzejewska, I. Zajdel, P. Pietrasik, E. Barsova, Z. & Goryczka, T. (2018). Application of X-ray powder diffraction and differential scanning calorimetry for identification of counterfeit drugs. 977-985.
View at Publisher | View at Google Scholar - Borer, K. T. (2005). Physical activity in the prevention and amelioration of osteoporosis in women: interaction of mechanical, hormonal and dietary factors. 779-830.
View at Publisher | View at Google Scholar - Weston, J. M. Norris, E. V. & Clark, E. M. (2011). The invisible disease: making sense of an osteoporosis diagnosis in older age. 1692-1704.
View at Publisher | View at Google Scholar - van Oostwaard, M. (2018). in Fragility Fracture Nursing: Holistic Care and Management of the Orthogeriatric Patient (eds K. Hertz & J. Santy-Tomlinson) 1-13 (Springer Copyright.
View at Publisher | View at Google Scholar - Lane, J. M. Russell, L. & Khan, S. N. (2000). Osteoporosis. 139-150
View at Publisher | View at Google Scholar - Szamatowicz, M. (2016). How can gynaecologists cope with the silent killer - osteoporosis? 189-192.
View at Publisher | View at Google Scholar - Diagnosis and treatment of osteoporotic fractures. (2009). 251-257.
View at Publisher | View at Google Scholar - Papaioannou, A. et al. (2002). Diagnosis and management of vertebral fractures in elderly adults. 220-228.
View at Publisher | View at Google Scholar - Glaser, D. L. & Kaplan, F. S. (1997). Osteoporosis. Definition and clinical presentation. Spine. 12-16.
View at Publisher | View at Google Scholar - Aslan, S. et al. (2005). Speed bump-induced spinal column injury. 563-564.
View at Publisher | View at Google Scholar - Siminoski, K. Warshawski, R. S. Jen, H. & Lee, K. (2006). The accuracy of historical height loss for the detection of vertebral fractures in postmenopausal women. Osteoporos. 290-296.
View at Publisher | View at Google Scholar - Alexandru, D. & So, W. (2012). Evaluation and management of vertebral compression fractures. 46-51.
View at Publisher | View at Google Scholar - Ensrud, K. E. Black, D. M. Harris, F. Ettinger, B. (1997). & Cummings, S. R. Correlates of kyphosis in older women. The Fracture Intervention Trial Research Group. J Am Geriatr 682-687.
View at Publisher | View at Google Scholar - Mumtaz, M. (2001). An approach to the patient with osteoporosis. 11-19.
View at Publisher | View at Google Scholar - Chou, S. H. & LeBoff, M. S. (2017). Vertebral Imaging in the Diagnosis of Osteoporosis: A Clinician's Perspective. 509-520.
View at Publisher | View at Google Scholar - Slemenda, C. W. Hui, S. L. Longcope, C. Wellman, H. & Johnston, C. C. Jr. (1990). Predictors of bone mass in perimenopausal women. A prospective study of clinical data using photon absorptiometry. 96-101
View at Publisher | View at Google Scholar - Kling, J. M. Clarke, B. L. & Sandhu, N. P. (2014). Osteoporosis prevention, screening, and treatment: a review.563-572.
View at Publisher | View at Google Scholar - Grados, F. et al. (2009). Radiographic methods for evaluating osteoporotic vertebral fractures. 241-247.
View at Publisher | View at Google Scholar - Genant, H. K. Wu, C. Y. van Kuijk, C. & Nevitt, M. C. (1993). Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 8:1137-1148.
View at Publisher | View at Google Scholar - Campbell, S. E. Phillips, C. D. Dubovsky, E. Cail, W. S. & Omary, R. A. (1995). The value of CT in determining potential instability of simple wedge-compression fractures of the lumbar spine. AJNR Am J Neuroradiol 16:1385-1392.
View at Publisher | View at Google Scholar - Sözen, T. Özışık, L. & Başaran, N. (2017). An overview and management of osteoporosis. Eur J Rheumatol. 4:46-56.
View at Publisher | View at Google Scholar - Camacho, P. M. et al. (2016). AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY CLINICAL PRACTICE GUIDELINES FOR THE DIAGNOSIS AND TREATMENT OF POSTMENOPAUSAL OSTEOPOROSIS. 22:1-42.
View at Publisher | View at Google Scholar - Chin, D. K. et al. (2007). Prevalence of osteoporosis in patients requiring spine surgery: incidence and significance of osteoporosis in spine disease. Osteoporos Int. 18:1219-1224.
View at Publisher | View at Google Scholar - Khalid, S. I. et al. (2020). Association of osteopenia and osteoporosis with higher rates of pseudarthrosis and revision surgery in adult patients undergoing single-level lumbar fusion.
View at Publisher | View at Google Scholar - Carlson, B. C. et al. (20019). A Review and Clinical Perspective of the Impact of Osteoporosis on the Spine. Geriatr Orthop Surg Rehabil.
View at Publisher | View at Google Scholar - Allen, R. T. Lee, Y.-P. & Garfin, S. R. (2009). Spine surgeons survey on attitudes regarding osteoporosis and osteomalacia screening and treatment for fractures, fusion surgery, and pseudoarthrosis. The Spine. 602-604.
View at Publisher | View at Google Scholar - Kalb, S. et al. (2013). Pharmacophysiology of bone and spinal fusion. Spine J 13: 1359-1369.
View at Publisher | View at Google Scholar - Dell, R. M. Greene, D. Anderson, D. & Williams, K. (2009). Osteoporosis disease management: What every orthopaedic surgeon should know. J Bone Joint Surg. (91) 6:79-86.
View at Publisher | View at Google Scholar - Pantoja, S. & Molina, M. (2019). Surgeon Management of Osteoporosis in Instrumented Spine Surgery: AOSpine Latin America Survey. Global Spine J. 9:169-172.
View at Publisher | View at Google Scholar - Ganesan, K. Jandu, J. S. Anastasopoulou, C. Ahsun, S. & Roane, D. (2022). in StatPearls (StatPearls Publishin Copyright © 2022, StatPearls Publishing LLC.
View at Publisher | View at Google Scholar - Avin, K. G. et al. (2022). Essential Components of Physical Therapist Management of Patients with Osteoporosis: A Delphi Study. J Geriatr Phys Ther. 45:120-126.
View at Publisher | View at Google Scholar - Preisinger, E. [Physical therapy in osteoporosis]. Wien Med Wochenschr 144, 612-618 (1994).
View at Publisher | View at Google Scholar - Metzger, M. F. Kanim, L. E. Zhao, L. Robinson, S. T. & Delamarter, R. B. (2015). The relationship between serum vitamin D levels and spinal fusion success: a quantitative analysis. Spine. 40:458-468.
View at Publisher | View at Google Scholar - Cho, J. H. et al. (2012). Effect of dietary calcium on spinal bone fusion in an ovariectomized rat model. J Korean Neurosurg Soc. 52:281-287.
View at Publisher | View at Google Scholar - Lehman, R. A., Jr. et al. The effect of alendronate sodium on spinal fusion: a rabbit model. Spine J 4, 36-43, doi:10.1016/s1529-9430(03)00427-3 (2004).
View at Publisher | View at Google Scholar - Nagahama, K. Kanayama, M. Togawa, D. Hashimoto, T. & Minami, A. (2011). Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J Neurosurg Spine. 14:500-507.
View at Publisher | View at Google Scholar - O'Loughlin, P. F. et al. (2009). Parathyroid hormone (1-34) augments spinal fusion, fusion mass volume, and fusion mass quality in a rabbit spinal fusion model. Spine. 34:121-130.
View at Publisher | View at Google Scholar - Lehman, R. A. J. et al. (2010). Effect of Teriparatide [rhPTH (1,34)] and Calcitonin on Intertransverse Process Fusion in a Rabbit Model. Spine. 35:146-152.
View at Publisher | View at Google Scholar - Abe, Y. et al. (2007). Enhancement of graft bone healing by intermittent administration of human parathyroid hormone (1–34) in a rat spinal arthrodesis model. Bone. 41:775-785.
View at Publisher | View at Google Scholar - Keaveny, T. M. et al. (2007). Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res. 22:149-157.
View at Publisher | View at Google Scholar - Nevitt, M. C. et al. (2006). Reduced risk of back pain following teriparatide treatment: a meta-analysis. Osteoporos Int. 17:273-280.
View at Publisher | View at Google Scholar - Gardner, M. J. Demetrakopoulos, D. Shindle, M. K. Griffith, M. H. & Lane, J. M. (2006). Osteoporosis and Skeletal Fractures. 2:62-69.
View at Publisher | View at Google Scholar - Lane, J. M. (1997). Osteoporosis: Medical Prevention and Treatment. Spine. 22:32-37.
View at Publisher | View at Google Scholar - Lane, J. M. Gardner, M. J. Lin, J. T. van der Meulen, M. C. & Myers, E. (2003). The aging spine: new technologies and therapeutics for the osteoporotic spine. European Spine Journal. 12:147-154.
View at Publisher | View at Google Scholar - Ettinger, B. et al. (1999). Reduction of Vertebral Fracture Risk in Postmenopausal Women with Osteoporosis Treated with RaloxifeneResults From a 3-Year Randomized Clinical Trial. 282:637-645.
View at Publisher | View at Google Scholar - Sarkar, S. et al. (2002). Relationships Between Bone Mineral Density and Incident Vertebral Fracture Risk with Raloxifene Therapy. Journal of Bone and Mineral Research. 17:1-10.
View at Publisher | View at Google Scholar - Chesnut, C. H. et al. (2000). A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. The American Journal of Medicine. 109:267-276.
View at Publisher | View at Google Scholar - Liu, Y. et al. (2012). Calcitonin enhanced lumbar spinal fusion in a New Zealand rabbit model: a study with morphologic and molecular analysis. Spine. 37:139-146.
View at Publisher | View at Google Scholar - Eastell, R. et al. (2011). Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res. 26:530-537.
View at Publisher | View at Google Scholar - Lehman, R. A. J. et al. (2003). Straight-Forward Versus Anatomic Trajectory Technique of Thoracic Pedicle Screw Fixation: A Biomechanical Analysis. Spine 28:2058-2065.
View at Publisher | View at Google Scholar - Dodwad, S. M. & Khan, S. N. (2013). Surgical stabilization of the spine in the osteoporotic patient. Orthop Clin North Am 44:243-249.
View at Publisher | View at Google Scholar - Choma, T. J. Pfeiffer, F. M. Swope, R. W. & Hirner, J. P. (2012). Pedicle Screw Design and Cement Augmentation in Osteoporotic Vertebrae: Effects of Fenestrations and Cement Viscosity on Fixation and Extraction. Spine. 37:1628-1632.
View at Publisher | View at Google Scholar - Chen, L. H. et al. (2011). Pullout strength of pedicle screws with cement augmentation in severe osteoporosis: a comparative study between cannulated screws with cement injection and solid screws with cement pre-filling. BMC Musculoskeletal Disorder 12.
View at Publisher | View at Google Scholar - Pfeifer, B. A. Krag, M. H. & Johnson, C. (1994). Repair of failed transpedicle screw fixation. A biomechanical study comparing polymethylmethacrylate, milled bone, and matchstick bone reconstruction. Spine. 19:350-353.
View at Publisher | View at Google Scholar - Sawakami, K. et al. (2012). Polymethylmethacrylate augmentation of pedicle screws increases the initial fixation in osteoporotic spine patients. J Spinal Disord Tech. 25:28-35.
View at Publisher | View at Google Scholar - Gazzeri, R. Roperto, R. & Fiore, C. (2012). Litanium expandable pedicle screw for the treatment of degenerative and traumatic spinal diseases in osteoporotic patients: preliminary experience. Surg Technol Int. 22:320-325.
View at Publisher | View at Google Scholar - Wu, Z.-x. et al. (2012). A comparative study on screw loosening in osteoporotic lumbar spine fusion between expandable and conventional pedicle screws. Archives of Orthopaedic and Trauma Surgery.s 132:471-476.
View at Publisher | View at Google Scholar - Anderson, P. A. Froyshteter, A. B. & Tontz, W. L. Jr. (2013). Meta-analysis of vertebral augmentation compared with conservative treatment for osteoporotic spinal fractures. J Bone Miner Res 28:372-382.
View at Publisher | View at Google Scholar - Wenger, M. & Markwalder, T. M. (2008). Vertebroplasty combined with pedicular instrumentation. J Clin Neurosci. 15:257-262.
View at Publisher | View at Google Scholar - Bogaert, L. et al. (2019). Postoperative bracing after lumbar surgery: a survey amongst spinal surgeons in Belgium. Eur Spine J. 28:442-449.
View at Publisher | View at Google Scholar - Soliman, H. A. G. et al. (2018). Early Impact of Postoperative Bracing on Pain and Quality of Life After Posterior Instrumented Fusion for Lumbar Degenerative Conditions: A Randomized Trial. Spine (Phila Pa 1976) 43:155-160.
View at Publisher | View at Google Scholar - Skoch, J. et al. (2016). Bracing After Surgical Stabilization of Thoracolumbar Fractures: A Systematic Review of Evidence, Indications, and Practices. World Neurosurg. 93:221-228.
View at Publisher | View at Google Scholar - Nasi, D. Dobran, M. & Pavesi, G. (2020). The efficacy of postoperative bracing after spine surgery for lumbar degenerative diseases: a systematic review. Eur Spine J 29:321-331.
View at Publisher | View at Google Scholar - Zhu, M. P. Tetreault, L. A. Sorefan-Mangou, F. Garwood, P. & Wilson J. R. (2013). Efficacy, safety, and economics of bracing after spine surgery: a systematic review of the literature. Spine J 181513-1525.
View at Publisher | View at Google Scholar - Kweh, B. T. S. et al. (2021). The Role of Spinal Orthoses in Osteoporotic Vertebral Fractures of the Elderly Population (Age 60 Years or Older): Systematic Review. Global Spine J 11:975-987.
View at Publisher | View at Google Scholar - Govindarajan, V. et al. (2021). Osteoporosis treatment in patients undergoing spinal fusion: a systematic review and meta-analysis. Neurosurgical Focus FOC 50:9.
View at Publisher | View at Google Scholar - Nicksic, P. J. et al. (2022). Electronic Bone Growth Stimulators for Augmentation of Osteogenesis in In Vitro and In Vivo Models: A Narrative Review of Electrical Stimulation Mechanisms and Device Specifications. Front Bioeng Biotechnol 10: 793945.
View at Publisher | View at Google Scholar - Aleem, I. S. et al. (2016). Efficacy of Electrical Stimulators for Bone Healing: A Meta-Analysis of Randomized Sham-Controlled Trials. Sci Rep 6:31724.
View at Publisher | View at Google Scholar - Park, P. Lau, D. Brodt, E. D. & Dettori, J. R. (2014). Electrical stimulation to enhance spinal fusion: a systematic review. Evid Based Spine Care J 5:87-94.
View at Publisher | View at Google Scholar - Griffin, M. & Bayat, A. Electrical stimulation in bone healing: critical analysis by evaluating levels of evidence. Eplasty 11:34 (2011).
View at Publisher | View at Google Scholar - Gupta, A. et al. (2021). Osteoporosis increases the likelihood of revision surgery following a long spinal fusion for adult spinal deformity. Spine J 21:134-140.
View at Publisher | View at Google Scholar

 Clinic
Clinic
