Review Article | DOI: https://doi.org/10.31579/2834-8389/018
Nasopharynaeal Adenoid Cystic Carcinoma, a Rare Disease: a Case Report and Review of Literature Medical Oncology Department of Annaba University Hospital
- Ifreke Okon Akpan
- Ifeanyichukwu Edeh *
Department of Chemical Engineering, University of Port Harcourt, Nigeria
*Corresponding Author: Ifeanyichukwu Edeh, Department of Chemical Engineering, University of Port Harcourt, Nigeria.
Citation: Ifreke Okon Akpan and Ifeanyichukwu Edeh, (2024), Nasopharynaeal Adenoid Cystic Carcinoma, a Rare Disease: a Case Report and Review of Literature Medical Oncology Department of Annaba University Hospital, International Journal of Clinical Case Reports, 3(2); DOI:10.31579/2834-8389/018
Copyright: © 2024, Ifeanyichukwu Edeh. This is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 01 April 2024 | Accepted: 11 April 2024 | Published: 24 April 2024
Keywords: biodiesel; transesterification; economic; feedstock; catalyst
Abstract
The current focus on renewable energy as a means of mitigating carbon footprint and emission of greenhouse gases has gathered momentum over the years. Biodiesel is one of the promising alternatives for the replacement of the conventional diesel. Currently about 36 billion liters of biodiesel has been produced globally by different countries using various feedstock such as edible oils, non-edible oils, algae oil, genetically modified microbes and waste sludge oils. Several techniques such as direct blending, microemulsion, thermal cracking and transesterification etc, have been used for production of biodiesel from various feedstock. The measure of the effectiveness of any technique depends on ease of operability and the percentage yield obtained at the end of the production process. Economic feasibility studies and life cycle assessment of biodiesel showed positive outcome indicating that biodiesel production and utilization is viable and sustainable.
Introduction
The rapid increase in the world population coupled with the advent of modern facilities has put much pressure on energy utilization. Record by Press (2015) indicated that the world total primary energy consumed (TPEC) as at 2015 was over 150,000,000 Gwh and it is estimated that by 2050 a rise of 57 % would be recorded. Over 80 % of this primary energy is from fossil fuel with crude oil leading with (35 %), coal ( 29%) and natural gas (24 %) (Baskar et al., 2017). According to report by Sieminski and Administrator (2016), 54 % of this, is utilized by transportation sector and is expected to increase by 1.1 % per year. The combustion of this fossil fuel has posed serious problem to the ecosystem. Report by Jamil et al. (2018) indicated that carbon dioxide emission would increase by 35
2.0 Feedstock for biodiesel production
Oils for biodiesel production are categorized into two major group edible and non- edible oils. Though the current trend in raw materials demands that non–edible oil be used as feedstock for biodiesel production to reduce food crises occasioned by the use of edible oil (Balat, 2011). Edible oils are obtained from edible sources mainly seeds and vegetables food sources. Some common edible oil feedstock are peanut, coconut, soybean, palm, rapeseed, sunflower oils etc. These oils give high percentage yield when used for biodiesel production. Over 0.82 liter of biodiesel can be obtained from 1litre of palm oil (Riazi et al, 2017). According to Karmakar et al. (2010), 1.3 liter of Soybean oil is capable of producing 1litre of biodiesel under a good processing procedure. Demirbas (2007) reported that 1.1litre of rapeseed oil can produce a yield of 1litre biodiesel under good production conditions. Non-edible oils are not used as food by human; most of them are poisonous why others are not hygienic for human consumption. Non-edible oils are derived from animals and plants sources including rubber seed oil, linseed oil, jatropha seed oil, karaja oil, tallow oil, castor oil, waste-cooking oil (Rincon et al., 2014). Most of these sources have high percentage of oil and promising qualities when use in biodiesel production. Jatropha seed contained about 35 – 60 wt% of oil and is very good for biodiesel synthesis. According to Acquage et al. (2012) about 89 % of biodiesel produced in UK is from waste- cooking oil. Animal fat is another promising feedstock for biodiesel because it reduces the use of edible oils for processing of biodiesel (Thamsiriroj et al., 2011). Algae have about 20 % to 80 % oil content depending on the species (Akubude et al 2016). Sewage sludge including activated sludge is also a promising feedstock for biodiesel production due to their lipid deposit (Edeh, 2019; Edeh et al., 2019a; Edeh et al, 2019b; Matthew et al, 2021).
3.0 Classifications of biodiesel
Biodiesel are classified based on era in which the particular feedstock was massively used. Three different generations are identified by Zulqarnain et al. (2021). These are first generation, second generation and third generation. The first generation feedstocks are from edible sources. The second generation biodiesel are produced from non-edible oils, and the third generations are made from micro-and macro-species like algae. The fatty acid compositions of the generations of biodiesel are presented in Table 2. Apart from the three common generations of biodiesel mentioned, the fourth generation has also been identified to be made from genetically modified algae (Edeh, 2020). This modified algae also promotes carbon dioxide capturing and utilization for algae production.
4.0 Reduction of Free Fatty Acid from Oil (Pretreatment)
Obviously, most of the oils for biodiesel production come with some percentage impurity. Some oils have high percentage of free fatty acid which can form soap during biodiesel synthesis, thereby reducing the yield of the product, thus the need for pretreatment. The two methods used to pretreat oils are the use of acid and glycerolysis. Acids such as H2SO4, H3PO4, HCl etc. are used to reduce free fatty content in oils. Hayyan et al. (2011) investigated the use of H2SO4 to reduce the free fatty acid and recorded reduction from 24 wt% to 2 wt%. Chai et al. (2014) using 10 wt% of H2SO4 reduced free fatty acid in waste-cooking oil from 5 wt% to 0.5 wt%. Three different acids (H2SO4, H3PO4 and HCl) were examined to ascertain their effectiveness in reducing free fatty acid content of waste-cooking oil by Sadaf et al. (2018). The result showed that H2SO4 was the most effective with reduction from 2.75 wt% to 0.33 wt% under the following reaction conditions, methanol to oil ratio of 2.5:1 and reaction temperature of 60 oC. Bolonio et al. (2019) established that the used of acid for the reduction of free fatty acid corrode the equipment and also consumed large amount of alcohol, thus it becomes imperative to find new methods in which free fatty acid reduction can be achieved. Glycerolysis reduced free fatty acid without causing corrosion to the reacting system. Glycerolysis has the advantage of increasing the quantity of glyceride in the feedstock while reducing the quantity of free fatty acid (Dias et al. 2017). Two different catalysts sodium sulfate (Na2SO4) and Zinc-aluminum oxide (Zn-Al2O3) were studied by Anderson et al. (2016) to ascertain their efficiency in reducing free fatty acid content of scum derive oil. The result showed that Zinc–Aluminum oxide was more effective by reducing free fatty acid from 86 wt% to 1 wt%.
| Classification | Type of feedstock | Palmitic acid (C16H32O2) | Stearic acid (C18H36O2) | Oleic acid (C18H34O2) | Linoleic acid (C18H32O2) | Linolenic acid (C18H30O2) |
First generation feedstocks | Soybean oil | 10.4–24.8 | 2.6–4.7 | 16.5–24.8 | 51.8–53.0 | 6.5–7.0 |
| Palm oil | 37.80–43.79 | 2.7–4.76 | 39.90–42.6 | 9.59–12.20 | 0.17–0.53 | |
| Olive oil | 9.7 | 1.74 | 82.3 | - | - | |
| Rapeseed oil | 3.49–4.0 | 0.55–2.3 | 62–77.8 | 1.8–8.23 | 1.8–8.23 | |
| Sunflower oil | 10.58 | 4.76 | 22.52 | 8.19 | 8.19 | |
Second generation feedstocks | Tallow oil | 29.0 | 24.5 | 44.5 | - | - |
| Jatropha. C oil | 14.2 | 7.0 | 44.7 | 32.8 | - | |
| P. pinnata oil | 10.2 | 7.0 | 51.8 | 17.7 | 0.2 | |
| M. indica oil | 24.5 | 22.7 | 37.0 | 14.3 | 3.6 | |
| Neem oil | 13.8 | 18.2 | 52.6 | 13.6 | - | |
| Rubber seed oil | 9.1 | 5.6 | 24.0 | 46.2 | 14.2 | |
| Linseed oil | 5.61 | 4.04 | 19.34 | 17.15 | 48.79 | |
| Castor oil | 0.92 | 0.16 | 3.53 | 4.21 | 0.91 | |
| Mustard oil | 2.80 | 1.09 | 24.98 | 11.64 | 8.61 | |
Third generation feedstocks | Crude castor oil | 1.06 | 1.15 | 3.71 | 5.41 | 0.58 |
| WCO | 4.1–26.5 | 4–10.9 | 38.6–44.7 | 32.8–36 | 0.2 | |
| Chicken fat oil | 19.82 | - | 37.62 | - | 1.45 | |
| Yellow grease | 23.24 | - | 44.32 | 2.43 | 0.80 | |
| Waste fryingoil | 6.90 | 2.35 | 61.58 | 20.01 | 20.01 | |
Waste animal fat,
| 22.31 | 17.02 | 43.26 | 9.76 | 1.71 |
Table 2: Compositions of various feedstock (%)
5.0 Oil Extraction
There are several methods that are applied for oil extraction. These methods are based on the source in which the oil is to be extracted.
Summary of some important methods of extraction are highlighted in Table 3.
| Technique | Merit | Demerit |
| Steam distillation | It is good for non- temperature sensitive plant | Continuous wetting is needed to supply the cold temperature feed Into the system. |
| Thermal degradation is minimized | It is capital intensive | |
| Solvent extraction | Small amount of solvent is needed to extract a large quantity of oil | Some of the solvent used are highly flammable, thus not environment friendly. |
| The process is easier to handle | ||
| Mechanical extraction | Oil extraction efficient using this method is better than manual | This method is limited to few oil sources. |
| Enzymatic extraction | It does not have negative effect on the environment | Enzymes used for this process are very expensive |
| Microwave-assisted extraction | CO2 is not released during this process | It can only be applied for non-polar compound |
| Supercritical fluids extraction | Due to high solubility of solvent with oil, good percentage of oil is obtained. | This technique requires high temperature, thus high cost of energy. |
| Ultrasound-assisted extraction | This method is not capital intensive but yield good percentage of oil. | It can cause degradation of the oil. |
Table 3. Summary of Oil Extraction Techniques for biodiesel production (Zulqarnain et al., 2020)
6.0 Biodiesel Production Techniques
There are different techniques by which biodiesel can be produced; some of the methods are highlighted below.
1.Direct Blending
To reduce the consumption of fossil fuel, crude oil from animal or plant source is blend directly with conventional diesel. Percentage ratio of mixing is very critical in achieving a good quality diesel. However, the biodiesel obtained from this method have some limitation such as high free fatty acid content, high viscosity and ease of gum formation. These make the biodiesel produced from this method unfit for many combustion engines (Chai et al, 2014).
2.Microemulsion
In this method, the oil is mixed with alcohol such as methanol, ethanol, propanol and butanol which are considered to be emulsifying agents. Other additives such as surfactant and alkyl nitrate are added to reduce viscosity, increase the cetane number and the volatile property of the diesel. The major problems associated with biodiesel produced via microemulsion include incomplete combustion, and accumulation of carbon in the engine which can lead to nozzle failure (Demirbas, 2009).
3.Thermal cracking (pyrolysis)
This is the application of heat in the absence of air to convert complex structure of hydrocarbon into simple compound with or without the aid of catalyst. This process helps in reducing density and viscosity of the biodiesel which are major parameters affecting combustion of biodiesel in diesel engines. Biodiesel obtained from this technique can be use directly without much modification. Pyrolysis process for biodiesel production is always performed between 250 0C and 350 0C with the use of catalyst such as alumina, and zeolite. the process is carryout in a reactor where the oil is been heated and then the vapour condensed to obtained biodiesel (Kansedo et al., 2009).
3.0 Classifications of biodiesel
Biodiesel are classified based on era in which the particular feedstock was massively used. Three different generations are identified by Zulqarnain et al. (2021). These are first generation, second generation and third generation. The first generation feedstocks are from edible sources. The second generation biodiesel are produced from non-edible oils, and the third generations are made from micro-and macro-species like algae. The fatty acid compositions of the generations of biodiesel are presented in Table 2. Apart from the three common generations of biodiesel mentioned, the fourth generation has also been identified to be made from genetically modified algae (Edeh, 2020). This modified algae also promotes carbon dioxide capturing and utilization for algae production.
4.0 Reduction of Free Fatty Acid from Oil (Pretreatment)
Obviously, most of the oils for biodiesel production come with some percentage impurity. Some oils have high percentage of free fatty acid which can form soap during biodiesel synthesis, thereby reducing the yield of the product, thus the need for pretreatment. The two methods used to pretreat oils are the use of acid and glycerolysis. Acids such as H2SO4, H3PO4, HCl etc. are used to reduce free fatty content in oils. Hayyan et al. (2011) investigated the use of H2SO4 to reduce the free fatty acid and recorded reduction from 24 wt% to 2 wt%. Chai et al. (2014) using 10 wt% of H2SO4 reduced free fatty acid in waste-cooking oil from 5 wt% to 0.5 wt%. Three different acids (H2SO4, H3PO4 and HCl) were examined to ascertain their effectiveness in reducing free fatty acid content of waste-cooking oil by Sadaf et al. (2018). The result showed that H2SO4 was the most effective with reduction from 2.75 wt% to 0.33 wt% under the following reaction conditions, methanol to oil ratio of 2.5:1 and reaction temperature of 60 oC. Bolonio et al. (2019) established that the used of acid for the reduction of free fatty acid corrode the equipment and also consumed large amount of alcohol, thus it becomes imperative to find new methods in which free fatty acid reduction can be achieved. Glycerolysis reduced free fatty acid without causing corrosion to the reacting system. Glycerolysis has the advantage of increasing the quantity of glyceride in the feedstock while reducing the quantity of free fatty acid (Dias et al. 2017). Two different catalysts sodium sulfate (Na2SO4) and Zinc-aluminum oxide (Zn-Al2O3) were studied by Anderson et al. (2016) to ascertain their efficiency in reducing free fatty acid content of scum derive oil. The result showed that Zinc–Aluminum oxide was more effective by reducing free fatty acid from 86 wt% to 1 wt%.
| Classification | Type of feedstock | Palmitic acid (C16H32O2) | Stearic acid (C18H36O2) | Oleic acid (C18H34O2) | Linoleic acid (C18H32O2) | Linolenic acid (C18H30O2) |
First generation feedstocks | Soybean oil | 10.4–24.8 | 2.6–4.7 | 16.5–24.8 | 51.8–53.0 | 6.5–7.0 |
| Palm oil | 37.80–43.79 | 2.7–4.76 | 39.90–42.6 | 9.59–12.20 | 0.17–0.53 | |
| Olive oil | 9.7 | 1.74 | 82.3 | - | - | |
| Rapeseed oil | 3.49–4.0 | 0.55–2.3 | 62–77.8 | 1.8–8.23 | 1.8–8.23 | |
| Sunflower oil | 10.58 | 4.76 | 22.52 | 8.19 | 8.19 | |
Second generation feedstocks | Tallow oil | 29.0 | 24.5 | 44.5 | - | - |
| Jatropha. C oil | 14.2 | 7.0 | 44.7 | 32.8 | - | |
| P. pinnata oil | 10.2 | 7.0 | 51.8 | 17.7 | 0.2 | |
| M. indica oil | 24.5 | 22.7 | 37.0 | 14.3 | 3.6 | |
| Neem oil | 13.8 | 18.2 | 52.6 | 13.6 | - | |
| Rubber seed oil | 9.1 | 5.6 | 24.0 | 46.2 | 14.2 | |
| Linseed oil | 5.61 | 4.04 | 19.34 | 17.15 | 48.79 | |
| Castor oil | 0.92 | 0.16 | 3.53 | 4.21 | 0.91 | |
| Mustard oil | 2.80 | 1.09 | 24.98 | 11.64 | 8.61 | |
Third generation feedstocks | Crude castor oil | 1.06 | 1.15 | 3.71 | 5.41 | 0.58 |
| WCO | 4.1–26.5 | 4–10.9 | 38.6–44.7 | 32.8–36 | 0.2 | |
| Chicken fat oil | 19.82 | - | 37.62 | - | 1.45 | |
| Yellow grease | 23.24 | - | 44.32 | 2.43 | 0.80 | |
| Waste fryingoil | 6.90 | 2.35 | 61.58 | 20.01 | 20.01 | |
Waste animal fat,
| 22.31 | 17.02 | 43.26 | 9.76 | 1.71 |
Table 2: Compositions of various feedstock (%)
5.0 Oil Extraction
There are several methods that are applied for oil extraction. These methods are based on the source in which the oil is to be extracted.
Summary of some important methods of extraction are highlighted in Table 3.
| Technique | Merit | Demerit |
| Steam distillation | It is good for non- temperature sensitive plant | Continuous wetting is needed to supply the cold temperature feed Into the system. |
| Thermal degradation is minimized | It is capital intensive | |
| Solvent extraction | Small amount of solvent is needed to extract a large quantity of oil | Some of the solvent used are highly flammable, thus not environment friendly. |
| The process is easier to handle | ||
| Mechanical extraction | Oil extraction efficient using this method is better than manual | This method is limited to few oil sources. |
| Enzymatic extraction | It does not have negative effect on the environment | Enzymes used for this process are very expensive |
| Microwave-assisted extraction | CO2 is not released during this process | It can only be applied for non-polar compound |
| Supercritical fluids extraction | Due to high solubility of solvent with oil, good percentage of oil is obtained. | This technique requires high temperature, thus high cost of energy. |
| Ultrasound-assisted extraction | This method is not capital intensive but yield good percentage of oil. | It can cause degradation of the oil. |
Table 3. Summary of Oil Extraction Techniques for biodiesel production (Zulqarnain et al., 2020)
6.0 Biodiesel Production Techniques
There are different techniques by which biodiesel can be produced; some of the methods are highlighted below.
1.Direct Blending
To reduce the consumption of fossil fuel, crude oil from animal or plant source is blend directly with conventional diesel. Percentage ratio of mixing is very critical in achieving a good quality diesel. However, the biodiesel obtained from this method have some limitation such as high free fatty acid content, high viscosity and ease of gum formation. These make the biodiesel produced from this method unfit for many combustion engines (Chai et al, 2014).
2.Microemulsion
In this method, the oil is mixed with alcohol such as methanol, ethanol, propanol and butanol which are considered to be emulsifying agents. Other additives such as surfactant and alkyl nitrate are added to reduce viscosity, increase the cetane number and the volatile property of the diesel. The major problems associated with biodiesel produced via microemulsion include incomplete combustion, and accumulation of carbon in the engine which can lead to nozzle failure (Demirbas, 2009).
3.Thermal cracking (pyrolysis)
This is the application of heat in the absence of air to convert complex structure of hydrocarbon into simple compound with or without the aid of catalyst. This process helps in reducing density and viscosity of the biodiesel which are major parameters affecting combustion of biodiesel in diesel engines. Biodiesel obtained from this technique can be use directly without much modification. Pyrolysis process for biodiesel production is always performed between 250 0C and 350 0C with the use of catalyst such as alumina, and zeolite. the process is carryout in a reactor where the oil is been heated and then the vapour condensed to obtained biodiesel (Kansedo et al., 2009).
4. Transesterification
Transesterification or alcoholysis is a reaction by which triglyceride (TG) reacts with alcohol in nucleophilic manner to produce Fatty Acid Methyl Ester (FAME) and glycerol as by – product (see Figure 1). The reaction involves three steps (i) reduction of triglyceride to diglyceride (ii) reduction of the diglyceride to monoglyceride (iii) the conversion of monoglyceride to glycerol. At each step, ester is been formed, thus the overall reaction gives rise to three molecules of ester (Changmai et al, 2020). The reaction is aided by the use of catalyst and there are different categories of catalysts that can be used for this purpose (base catalyst, acid catalyst, enzyme, bifunctional catalyst etc). The choice of the catalyst depends on the free fatty acid content of the oil.
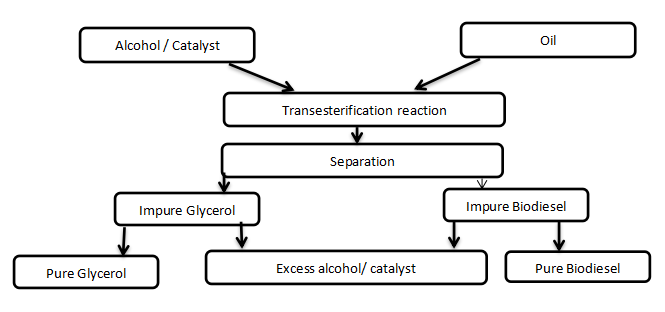
Figure 1 : Transesterification reaction scheme
7.0 Biodiesel production reactors
Reactors are equipment which provides an enabling condition for chemical reaction to occur. In a reactor, reactants are transformed to products after been subjected to the required conditions. There are many configurations (types) of reactors that are used for biodiesel synthesis, depending on the production method either in batch or continuous process (Gerpen, 2005). Below are some of the reactors used in biodiesel production
| Type of reactor | Description | Advantage | Disadvantage | References |
| Batch | It is a vessel with stirring system, thermometer and pressure gauge attached to it. | It is easy to operate | Poor control of mass transfer.
Bulky size of reactor | Kraai (2009)
Abdurakhman et al. (2017)
|
| Continuous Stirrer Tank Reactor | Reactants and product(s) flow in and out continuously. Parameters includes input and output rate, agitation speed, residence time, mass transfer rate and mixing efficiency.
| It produces quality product.
Operation parameters are easily control. | It required much energy.
High cost of operation.
| Janajreh et al.(2016)
|
Fixed Bed Reactor
| It is a cylindrical tube packed with some catalyst pellet in a fixed bed. . | It promotes longevity and activity of heterogeneous catalysis. Reaction time is reduced. | High ratio of alcohol to oil is required for optimum yield. Accumulation of the by-product on the surface of the catalyst. | Hama et al. (2013)
|
| Bubble Column Reactor | The reacting scheme of bubble column reactor is divided into two, vapour and liquid phase. Reaction occurred at very high temperature. | Reduces formation of soap.
Oil with high free fatty acid can be used without pretreatment. | biocatalyst cannot be used because there are easily destroy by mechanical agitation | Adeleke. (2021) |
Reactive Distillation Column
| In reactive distillation The product formation and removal occur concurrently. | The major advantage of using this process is the combination of reaction and separation unit in single system | Complex arrangement of equipment | Adeleke. (2021)
|
Hybrid Catalytic Plasma Reactor
| This reactor uses high energy electrons to energize reacting molecules. | It does not required catalyst, short resident time, no formation of soap. | The major challenge of the reactor is the regulation of the reaction parameter. | Istadi et al. (2014).
|
| Membrane reactor | Here the reaction and separation of product(s) via membrane separation occur simultaneously in the same apartment | It has the ability to regulating the mixing of the reacting molecules and also possesses high selectivity | Sensitive to temperature different | Vares et al. (2014) |
Sonochemical Reactor
| This is a novel reactor that utilized energy from ultrasonic irradiation to generate cavitation | Low operating cost , high yield and selectivity, operating conditions are easy to control | Gogate et al. (2009)
Colucci et al. (2005) |
Table 4: Type of biodiesel reactors
8.0 Biodiesel Reagents
The two major reagents for biodiesel production are alcohol and fatty acid (oil). Alcohol is an important raw material for biodiesel synthesis. Several alcohols have been investigated for biodiesel production, but the most widely used are methanol and ethanol. This is because of their physical and chemical properties which makes them very reactive with triglyceride (Demirbas, 2008). Other lower chain alcohol like propanol, butanol, isopropanol and branch chain alcohol are also investigated. The ratio of alcohol to oil is another important factor to be considered during transesterification reaction. It affects the yield, rate of the reaction and cost of production. According to Barnwal and Sharma (2005), the ratio of oil to methanol when alkali catalyst is used is 1: 6, the high amount of methanol is to break the chain of fatty acid – glycerol (Agarwal, 2007), thus alcohol to oil ratio above 6 : 1 influences the production process negatively, and does not increase the amount of biodiesel produce. Silva et al. (2011) reported incomplete reaction with ratio less than 6:1 while higher ratio above 15:1 resulted in difficult separation of products and subsequent decrease in percentage yield.
9.0 Catalysts used for biodiesel production
Catalyst create alternative pathway which allowed for the utilization of minimum amount energy thereby cutting down the cost of the process (Robert 2017). In the process, it increasing the rate of formation of the desired product and lower the formation of the undesired product, this is term selectivity. Catalysts used for biodiesel production are categorized into four groups and each category comprises of sub-groups, there are homogeneous, heterogeneous, immobilized enzymes and bifuncional categories.
(a). Homogeneous Catalysis
Homogeneous catalysts used for transesterification process are basically of two types; acid and base. These are the most widely used and investigated catalyst for biodiesel production because they are less expensive and properties are easy to study. The base catalysts are basically metallic hydroxides from group 1 and 2 elements (NaOH, KOH, NaOCl etc). Hassain and Boyce (2009) used KOH in pure sunflower cooking oil and waste sunflower cooking oil and reported yield of 99.5 % under the following reaction conditions 40 oC, stirring speed 320 rmp and methanol-to-oil ratio of 6:1. Buendia-Tamariz et al. (2015) synthesized biodiesel from chicken fat and pennycress oil via double steps method; in the first step, esterification of the oils were carried out using H2SO4 while second step, the transesterificattion make use of KOH catalyst. Homogeneous base catalyst gives a better yield than its acid counterpart but when oil feedstock with high free fatty acid is involved, the basic catalyst react to form soap via saponification and this would consumed the catalyst and reduces its activity thus some oils required acid catalyst. Daniyan et al. (2019) used HCl as catalyst for frying oil transesterificattion and reported yield of 93 %. Goff et al. (2004) carried out a study to evaluate alcoholysis of soybean oil using hydrochloric acid, formic acid, acetic acid, sulphuric acid and nitric acid and reported that sulphuric acid gave the maximum yield. Miao et al. (2009) investigated biodiesel production from soybean oil using trifluroacetic acid as catalyst. The result showed 98.4% conversion at optimal reaction conditions.
(b). Heterogeneous catalyst
Heterogeneous catalyst reduces process cost and improve the quality of product obtained in addition to been eco- friendly. Aside from the above mentioned advantages, it can be easily recover; can withstand harsh reaction conditions and has longer reusability than homogeneous catalyst. There are majorly two categories of heterogeneous catalyst, acid and base. Each of the categories comprise sub-groups which are derivative of the parent group. Acid heterogeneous catalysts are derived mainly from mineral acid; they showe high activity in catalyzing biodiesel production from oil with high value of free fatty acid without formation of soap. It is a good substitute for homogeneous acid catalyst because it is ease to separate, minimal corrosiveness and toxicity. Umar et al. (2019), investigated the potential of Amberlyst 15 (ion exchange resin) in catalyzing biodiesel production from vulgaris seed oil and reported 93.2 % yield. A report from the work of Kansedo et al. (2009) has it that 80 % yield of biodiesel was obtained from transesterification of hydrolyzed sea mango oil using Amberlyst 15. Deboni et al. (2018) produced biodiesel from soybean oil using Amberlyst 26 and reported 99 % yield of FAME. Ilgen et al. (2009) reported 63 % yield when Amberlyst 26 was used to catalyzed biodiesel production from canola oil under optimum conditions. Heterogeneous catalysts from basic sources are the most widely investigated catalyst because of its excellent catalytic activity under mild reaction conditions. Alkaline earth oxides are the bases of these catalysts due to its high basicity and insolubility in methanol. It can maintain its efficiency up to 10 cycles (Liu et al, 2008). Several heterogeneous solid base catalysts have been reported in many literatures. Kouzu et al. (2009), prepared and investigated CaO catalyst for production of biodiesel from soybean oil and reported 95 % conversion. Dahkhoda et al. (2010) produced biodiesel from palm oil using KOH/activated carbon catalyst and reported 94 % yield.
(c.) Sulfonated carbon- base catalysts
Sulfonated carbon is another potential catalyst that has shown a promising activity in transesterification reaction. This is basically due to its distinctive surface chemistry, high thermal and chemical stability aside from been biogenic and eco-friendly the feedstock are cheap. It can be prepared from different methods like incomplete carbonization of aromatic substances in tetraoxosulfate(vi)acid or treating carbon materials with sulfonated reagents like ClSO3H, P-toluenesulfonic acid, gaseous SO3 etc (Malins et al 2016). Nakajima et al. (2012) prepared amorphous cellulose originated carbon solid acid catalyst and used it for biodiesel production from oleic acid. The report has it that 99.9 % conversion was recorded.
(d). Zeolites
Zeolites are microporous crystalline of aluminosilcates. Depending on the preparation process, it can exist in different structural morphologies. The ease by which its structure can be modified makes them excellence catalyst for transesterification reaction. When the pore size and the ratio of Si/Al are varied, the catalyst properties vary too. Different metal ions can be incorporate to improve the basicity of the catalyst. Several Zeolites have been prepared and used as catalyst for transesterification reaction. Xie et al. (2007) synthesized and investigated zeolites load with different concentrations of KOH and recorded 85.6 % yield of biodiesel with 10 % KOH loaded zeolite. Shu et al. (2007) synthesized La/Zeolites catalyst using La(NO2)3 as precursor through ion exchange approach and reported 48.9 % biodiesel yield when used for transesterificaion of soybean oil.
(e.) Hydrotalicites
Another promising group of catalyst is the hydrotalicite. It belongs to the double layer hydroxides with the general formulae Mn2+ Mm3+(OH)2(m+n)[Ax-]m/x. YH2O where M2+ is a divalent metal and M3+ is a trivalent metal and often A3+,Ax- is an anion with x in the range of 0.1 – 0.5. Many of this catalyst are currently in used. Nayajas et al. (2018) synthesized Mg/Al hydrotalicite catalyst via co-precipitation method and utilized it in transesterification of sunflower oil and reported 96 % yield. Ma et al. (2016) applied Mg/Al hydrotalicite catalyst for biodiesel production from waste- cooking oil and reported a yield of 95.2 %. In another study by Zeng et al. (2014) Mg/Al-CO3 hydrotalcite catalyst was prepared with ratio of Mg/Al of 4:1 using Urea method. The report has it that conversion of 90.3 % biodiesel was obtained when the catalyst was used for transesterification of microalgae oil.
(f) Mixed metal oxides
It is obvious from many researches that when two metallic oxides of different basicity are combined, their individual properties changes, one would behave like an acid while the other assumed basic nature. It is on this light that mixed oxides are used for catalyst synthesis. Several catalysts of this series have been prepared with report of high catalytic activity, increased basicity, good surface area, high stability and reusability. Kawashima et al, (2008) examined different calcium containing catalyst (CaTiO3, CaMnO3, Ca2Fe2O5, CaZrO3 and CaO-CeO2) in transesterification of rapeseed oil and reported biodiesel yield of 90%. A mixed oxide of La2O3 loaded with ZrO2 was prepared by (Sun et al, 2010) and reported biodiesel yield of 96% with sunflower oil.
(g) Biomass - based catalyst
Recently waste from biomass has been used to synthesize catalysts for biofuel and other chemical processes. Their variable composition of different oxides makes them promising area for research. Its usage aside from helping in copping the menace of environmental pollution also provide less expensive, less toxic, ecofriendly, sustainable and easily available source of catalysts. They can be derive from both animals and plants wastes in industries and households. Many of them are good sources of alkaline and alkaline earth oxides. Several works have been reported on the formulation and usage of biomass–based catalysts for biodiesel production. Wei et al. (2009) and Goli and Sahu (2018) reported a preparation of CaO catalyst from chicken eggshell, when applied in transesterification of soybean oil with a yield of 95 % and 93% were recorded respectively. Ayodeji et al. (2018) formulated catalyst using eggshell for biodiesel synthesis from soybean oil. According to the report 90 % yield of biodiesel was obtained.
(h). Enzymes
Enzymes catalysts are another promising set of catalysts that showed great potential in catalyzing transesterification reaction. They are very reactive and produce good quality biodiesel under mild reaction conditions with ease of separability. They can catalyze oil feedstock with high free fatty acid content without formation of soap. The catalyst can be used in free form or in immobilize lipase form. Jayaraman et al. (2020) applied lipase as catalyst to synthesized biodiesel from waste-cooking oil and reported yield of 88%. Marin-suarez et al. (2019) using process optimization synthesized biodiesel from low quality fish oil using lipase catalyst.
(i). Bifunctional catalyst
Bifunctional heterogeneous catalysts are those types of catalyst that has both acidic and basic properties, they are prepared from oxides of either group one and three or two and three. The group three oxides been amphoteric in nature always assumed acidic properties while the other oxide shows basic properties. Thus, it can catalyze both esterification and transesterification simultaneously. They showed good activity during biodiesel process even with oil that has high fatty acid content. Its effectiveness in biodiesel production overshadowed the shortfall of both basic and acid catalysts. Farooq et al. (2013), prepared Mo-Mn/y-Al2O3-MgO bifunctional catalyst and used it to catalyzed wastes cooking-oil having free fatty acid content of 3.37 mgKOH/g and reported 91.4 % biodiesel yield. Sulaman et al. (2016), developed Cu-Zn/y-Al2O3 bifunctional catalyst and used it for simultaneous transterification and esterification of waste cooking oil and recorded biodiesel yield of 88.82 %.
- Factors affecting biodiesel production via transesterification
The yield of biodiesel is influenced by some factors like temperature of the reaction, time of reaction, methanol to oil ratio, mixing rate, effect of water and free fatty acid content. These factors depend on the type of catalyst used for the transesterification.
(i). Alcohol to oil ratio
The ratio of alcohol to oil is very critical in biodiesel synthesis and it influences the yield significantly. It depends mostly on the type of catalyst used for the process. For example, when base catalyst is used, the alcohol to oil ratio is fixed at 6:1 according to (Zhang et al, 2003). A report from Jain et al. (2014) has it that when the quantity of methanol was increased from 10 to 30 % (v/v), a high yield of 90.6 % was obtained. It is imperative to note that, transesterification been a reversible reaction, requires large amount of methanol to keep the equilibrium in the product side. Increasing the ratio above particular critical value does not promote the reaction rate or percentage yield (Yaqoob et al, 2020). Nevertheless, excess used of alcohol would increase the overall cost of production due to alcohol removal and purification of the product.
(ii). Temperature
Reaction temperature is one of the most important factors that affect biodiesel yield in transesteriffication reaction. Temperature increase speed–up the rate of reaction and reduces the reaction time due to reduction of viscosity of the oil (Mathiyazhagan et al., 2011). During reaction, the energy possess by the reacting molecules is proportional to temperature. Thus elevated temperature of the reaction would promote miscibility between non-polar oil and the polar alcohol. But the temperature has to be controlled in order not to exceed the maximum value that is necessary for the reaction because if it does, parallel reaction may occur which would decrease the yield of the biodiesel. Kumar et al., (2013) obtained optimum yield of 96 % biodiesel at 80 oC when using temperature range of 60 to 120 oC in transesterification of sunflower oil. In a similar work, Joshi et al. (2019) obtained optimum yield at 60 oC during transesterification using waste-cooking.
(iii). Reaction time
Reaction time is very essential in biodiesel synthesis; researches have been performed to ascertain optimum time for biodiesel production. Alamu et al. (2007) observed that the rate of conversion increases from the start to 90 mins after which no much effect was observed. Leung and Guo (2006) observed that longer reaction time beyond the peak lead to loss of biodiesel due to reversible reaction and formation of soap.
(iv). Amount of catalyst and type
Biodiesel yield during transesterification reaction depends largely on the type and amount of catalyst used. Utilizing large amount of catalyst promotes the rate of reaction and the subsequent increase in yield, this is due to the availability of more active site (Rashid, 2008). However, the use of excess catalyst can lead to high viscosity of the resultant biodiesel. Thus, amount of catalyst must be controlled to prevent high cost of purification. Rathore et al. (2015), observed that when the concentration of KOH catalyst was increase from 2 to 12 %, the biodiesel yield increases from 20 to 95 %. Biodiesel yield increases until optimum load of catalyst is used.
(v). Effect of water and FFA content
Water and free fatty acid content in reactants are critical in the choice of reaction route during transesterification. There are percentage limits of each of these (water and free fatty acid content) that can be allowed in feedstock before the reaction can be effective. For example base-catalyst can only be used in oil with free fatty acid content of less than 1 % to avoid formation of soap via saponification reaction. Similarly acid catalyst can lead to ester formation in oil with high water content. Nevertheless, transesterification that occur at supercritical condition does not required the removal of water and free fatty acid content from the oil.
(vi). Mixing intensity
Alcohols and oils are not perfectly soluble when mixed, thus at rest (when the mixture is not agitated) the reaction occurred at the interface between the two liquids. This makes transesterification reaction to be slow at the beginning. Intensity of mixing is very important factor during transesterification reaction because it allows for oil and solvent interaction which in turn increases the rate of reaction. Many literatures have acknowledged that moderate agitation speed of 400rmp gives the optimum yield Tabatabaei et al, 2019. It has been noted that lower agitation speed slow the rate of reaction while high agitation speed causes the formation soap due to irreversibility.
- Life Cycle Assessment (LCA)
Life Cycle Assessment is a means through which impact of process or product on the environment is evaluated. It assesses the raw material(s) acquisition, energy utilization, production process; transportation of raw materials and finish product, product usage and product disposal and this approach is called Cradle to Grave analysis. Knowing the impact of a product to the environment is very necessary because it helps in decision making process about the product either to improve on the production route or to abandon the utilization of the production. There are basically four stages in LCA and are goal and scope, inventory analysis, impact assessment and interpretation. Life cycle Assessment of biodiesel is very important because it help to ascertain the impact of biodiesel to the environment so that if the negative effect is much, the impact can be reduced or processes leading to such impact can be improved. LCA performed by Sebitso et al. (2015) on biodiesel using Simapro 7.3.3 as analyzing tool with waste cooking and NaOH as oil source and catalyst respectively. The outcome of the finding indicated that during transesterification process, electricity has the highest environmental impact followed by alcohol. The grand analysis showed that the use of van for the distribution of biodiesel had the highest environmental impact than any other stage. According to Liang et al. (2013), different feedstock have different environmental impact, but concluded that biodiesel usage mitigate environmental pollution as compare to fossil diesel.
- Economics feasibility
Economic feasibility study is an important aspect of production process because it helps to evaluate the economic validity of the final product whether it would yield profit or not. This is done after the pilot production has been optimized (Mathew et al, 2021). The feasibility study takes into consideration the cost of raw materials, transportation, energy, labour and equipment, sometime the cost of alternative product(s) in the market. Biodiesel economic feasibility studies have been performed by various researchers to ascertain the economic validity of the product. Using Aspen Hysys v 7.0 El-Galad et al. (2015) studied the production of biodiesel using fatty acid from oil and soap industries and concluded that biodiesel production using fatty acid from these is feasible and cheap with a yield of high quality biodiesel. Another study on the use of Pongamia oil for biodiesel synthesis performed by (Prasad et al., 2020) indicated positive Net Present value and Benefit Cost Ratio of greater than 1, which means that the production process is economically viable. Raw material and catalyst cost are major challenges in biodiesel production. According to Edeh (2020) this can be mitigated by using alternatives and advance technologies. Mizik et al. (2021) ascertain that the use of waste cooking oil or algae oil as feedstock is economically viable than other feedstock.
Conclusion
The use of biodiesel as alternative fuel has come to stay and it has been proven to be efficient, economically viable, means by which greenhouse gases emission can be mitigated as well as a good way of waste management. Various raw materials for its production are readily available and researches are ongoing for development of novel feedstock that will enhance optimum production. Likewise, several production techniques have emerged over the years and new ones are still emerging geared towards improving product yield and process efficiency. Its utilization as alternative source of energy cut across manufacturing and transportation sectors without modification of the combustion chamber of the engine. Life Cycle Assessment conducted by several researchers pointed at positive index from raw materials acquisition to product disposal (cradle to grave) and is economically sustainable. Thus, it is imperative for government and other regulatory bodies to encourage the production and utilization of biodiesel by providing the enabling environment for it to strive.
References
- 1. Abdallah, N. E, Ezeldin Osman, M., Sheshko, T. F., Dipheko, T. D., Hassan, E. A., & Ishak, C. Y. (2021). Synthesis and improvement of Jatropha curcas L. biodiesel based on eco-friendly materials. International Journal of Green Energy, 18(13), 1396-1404.
View at Publisher | View at Google Scholar - 1. Abdurakhman, Y. B., Putra, Z. A., & Bilad, M. R. (2017). Process simulation and economic analysis of biodiesel production from waste cooking oil with membrane bioreactor. In AIP Conference Proceedings 1891(1):020011. AIP Publishing LLC.
View at Publisher | View at Google Scholar - 1. Adeleke, A. A., Odusote, J. K., Lasode, O. A., Ikubanni, P. P., Madhurai, M., & Paswan, D. (2021). Evaluation of thermal decomposition characteristics and kinetic parameters of melina wood. Biofuels, 13(1), 117-123.
View at Publisher | View at Google Scholar - 2. Agarwal, A. K. (2007). Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Progress in energy and combustion science, 33(3), 233-271.
View at Publisher | View at Google Scholar - 3. Akubude, V. C., & Nwaigwe, K. N. (2016). Economic importance of edible and non-edible almond fruit as bioenergy material: a review. American Journal of Energy Science, 3(5), 31-39.
View at Publisher | View at Google Scholar - 4. Alamu, O. J., Waheed, M. A., Jekayinfa, S. O., & Akintola, T. A. (2007). Optimal t transesterification duration for biodiesel production from Nigerian palm kernel oil. Agricultural Engineering International: CIGR Journal.
View at Publisher | View at Google Scholar - 5. Anderson, E., Addy, M., Xie, Q., Ma, H., Liu, Y., Cheng, Y., ... & Ruan, R. (2016). Glycerin esterification of scum derived free fatty acids for biodiesel production. Bioresource technology, 200, 153-160.
View at Publisher | View at Google Scholar - 6. Ayodeji, A. A., Blessing, I. E., Rasheed, B., Modupe, O. E., Ajibola, O., Oluwabunmi, A. G., & Ojo, F. S. (2018). Production of biodiesel from soybean oil using calcium oxide and cow bone as catalysts. Materials Focus, 7(4), 542-548.
View at Publisher | View at Google Scholar - 7. Barnwal, B. K., & Sharma, M. P. (2005). Prospects of biodiesel production from vegetable oils in India. Renewable and sustainable energy reviews, 9(4), 363-378.
View at Publisher | View at Google Scholar - 8. Baskar, G., Aiswarya, R., Soumiya, S., Mohanapriya, N., & Nivetha, S. R. (2017). Recent advances in heterogeneous catalysts for biodiesel production. J. Energy Environ. Sustain, 4(1), 10-47469.
View at Publisher | View at Google Scholar - 9. Balat, M. (2011). Potential alternatives to edible oils for biodiesel production–A review of current work. Energy conversion and management, 52(2), 1479-1492.
View at Publisher | View at Google Scholar - 10. Bolonio, D., García-Martínez, M. J., Ortega, M. F., Lapuerta, M., Rodríguez-Fernández, J., & Canoira, L. (2019). Fatty acid ethyl esters (FAEEs) obtained from grapeseed oil: A fully renewable biofuel. Renewable Energy, 132, 278-283.
View at Publisher | View at Google Scholar - 11. Buendía-Tamariz, M. N., Trejo-Calzada, R., Abiola, A., Pedroza-Sandoval, A., Jacobo-Salcedo, M. R., & Reveles-Hernández, M. (2015). Characterization of Biodiesel Produced from Chicken Fat and Pennycress Oil using Different Concentrations of Basic Catalysts. Journal of Agriculture and Environmental Sciences, 4(1), 127-133.
View at Publisher | View at Google Scholar - 12. Chai, M., Tu, Q., Lu, M., & Yang, Y. J. (2014). Esterification pretreatment of free fatty acid in biodiesel production, from laboratory to industry. Fuel processing technology, 125, 106-113.
View at Publisher | View at Google Scholar - 13. Changmai, B., Sudarsanam, P., & Rokhum, L. (2020). Biodiesel production using a renewable mesoporous solid catalyst. Industrial crops and products, 145, 111911.
View at Publisher | View at Google Scholar - 14. Colucci, J. A., Borrero, E. E., & Alape, F. (2005). Biodiesel from an alkaline transesterification reaction of soybean oil using ultrasonic mixing. Journal of the American Oil Chemists' Society, 82, 525-530.
View at Publisher | View at Google Scholar - 15. Deboni, T. M., Hirata, G. A. M., Shimamoto, G. G., Tubino, M., & de Almeida Meirelles, A. J. (2018). Deacidification and ethyl biodiesel production from acid soybean oil using a strong anion exchange resin. Chemical Engineering Journal, 333, 686-696.
View at Publisher | View at Google Scholar - 16. Daniyan, I. A., Daniyan, L., Adeodu, A., Uchegbu, I. (2019). Performance evaluation of a Smart multi feedstock biodiesel plant. Procedia Manufacturing, 35: 1117-1122
View at Publisher | View at Google Scholar - 17. Demirbas, A. (2009). Progress and recent trends in biodiesel fuels. Energy conversion and management, 50(1), 14-34.
View at Publisher | View at Google Scholar - 18. Demirbas, A. (2007). Importance of biodiesel as transportation fuel. Energy policy, 35(9), 4661-4670.
View at Publisher | View at Google Scholar - 19. Dehkhoda, A. M., West, A. H., & Ellis, N. (2010). Biochar based solid acid catalyst for biodiesel production. Applied Catalysis A: General, 382(2), 197-204.
View at Publisher | View at Google Scholar - 20. Demirbas, A. (2008). Biofuels sources, biofuel policy, biofuel economy and globa biofuel projections. Energy conversion and management, 49(8), 2106-2116.
View at Publisher | View at Google Scholar - 21. Edeh I (2019). Activated Sludge for Bioenergy and Oleochemical Production. Germany: LAP LAMBERT Academic publishing. p. 304
View at Publisher | View at Google Scholar - 22. Edeh I, Overton T, Bowra S. (2019a) Evaluation of the efficacy of subcritical water to enhance the lipid fraction from activated sludge for biodiesel and oleochemical production. Journal of Food Process Engineering. 42:1-9. DOI: 10.1111/jfpe.13070
View at Publisher | View at Google Scholar - 23. Edeh I, Overton T, Bowra S. (2019b). Optimization of subcritical watermediated lipid extraction from activated sludge for biodiesel production. Biofuels. DOI: 10.1080/ 17597269.2018.1558839
View at Publisher | View at Google Scholar - 24. Edeh, I. (2020). Biodiesel Production as a Renewable Resource for the Potential Displacement of the Petroleum Diesel. In Biorefinery Concepts, Energy and Products. IntechOpen.
View at Publisher | View at Google Scholar - 25. El-Galad, M. I., El-Khatib, K. M., & Zaher, F. A. (2015). Economic feasibility study of biodiesel production by direct esterification of fatty acids from the oil and soap industrial sector. Egyptian journal of petroleum, 24(4), 455-460.
View at Publisher | View at Google Scholar - 26. Farooq, M., Ramli, A., & Subbarao, D. (2013). Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. Journal of Cleaner Production, 59, 131-140.
View at Publisher | View at Google Scholar - 27. Gerpen, V. J. (2005). Biodiesel processing and production. Fuel processing technology, 86(10), 1097-1107.
View at Publisher | View at Google Scholar - 28. Gogate, P. R. & Sutkar, V. S., (2009). Design aspects of sonochemical reactors: techniques for understanding cavitational activity distribution and effect of operating parameters. Chemical Engineering Journal, 155(1-2), 26-36.
View at Publisher | View at Google Scholar - 29. Goli, J., Sahu, O. (2018). Development of heterogeneous alkali catalyst from waste chicken eggshell for biodiesel production. Renewable Energy, 128, 142-154.
View at Publisher | View at Google Scholar - 30. Hama, S., Yoshida, A., Tamadani, N., Noda, H., & Kondo, A. (2013). Enzymatic production of biodiesel from waste cooking oil in a packed-bed reactor: an engineering approach to separation of hydrophilic impurities. Bioresource technology, 135, 417-421.
View at Publisher | View at Google Scholar - 31. Hayyan, A., Alam, M. Z., Mirghani, M. E., Kabbashi, N. A., Hakimi, N. I. N. M., Siran, Y. M., & Tahiruddin, S. (2011). Reduction of high content of free fatty acid in sludge palm oil via acid catalyst for biodiesel production. Fuel Processing Technology, 92(5), 920-924.
View at Publisher | View at Google Scholar - 32. Hossain, A. B. M. S., & Boyce, A. N. (2009). Biodiesel production from waste Sunflower cooking oil as an environmental recycling process and renewable energy. Bulgarian journal of agricultural science, 15(4), 312-317.
View at Publisher | View at Google Scholar - 33. İlgen, O., Akin, A. N., & Boz, N. (2009). Investigation of biodiesel production from canola oil using Amberlyst-26 as a catalyst. Turkish Journal of Chemistry, 33(2), 289-294.
View at Publisher | View at Google Scholar - 34. Istadi, I., Yudhistira, A. D., Anggoro, D. D., & Buchori, L. (2014). Electro-catalysis system for biodiesel synthesis from palm oil over dielectric-barrier discharge plasma reactor. Bulletin of Chemical Reaction Engineering & Catalysis, 9(2), 111.
View at Publisher | View at Google Scholar - 35. Janajreh, I, Hussain, M. N., Samad, T. E., &. Daqaq, M. (2016). Numerical modelling of sonicated, continuous transesterification and evaluation of reaction kinetics for optimizing biodiesel reactor design. Int. J. of Thermal & Environmental Engineering, 11(1), 79-86
View at Publisher | View at Google Scholar - 36. Jain, R., Mahto, T. K., Chandra, S., Roy, D., Mahto, V., & Sahu, S. K. (2014). Single step synthesis of sulfonic group bearing graphene oxide: a promising carbo-nano material for biodiesel production. Journal of environmental chemical engineering, 4(3), 2933-2940.
View at Publisher | View at Google Scholar - 37. Jamil, F., Myint, M. T. Z., Al-Hinai, M., Al-Haj, L., Baawain, M., Al-Abri, M., & Atabani, A. E. (2018). Biodiesel production by valorizing waste Phoenix dactylifera L. Kernel oil in the presence of synthesized heterogeneous metallic oxide catalyst (Mn@ MgO-ZrO2). Energy Conversion and Management, 155, 128-137.
View at Publisher | View at Google Scholar - 38. Jayaraman, J., Alagu, K., Appavu, P., Joy, N., Jayaram, P., & Mariadoss, A. (2020). Enzymatic production of biodiesel using lipase catalyst and testing of an unmodified compression ignition engine using its blends with diesel. Renewable Energy, 145, 399-407.
View at Publisher | View at Google Scholar - 39. Joshi, S., Hadiya, P., Shah, M., & Sircar, A. (2019). Techno-economical and experimental analysis of biodiesel production from used cooking oil. BioPhysical Economics and Resource Quality, 4, 1-6.
View at Publisher | View at Google Scholar - 40. Kansedo, J., Lee, K. T., & Bhatia, S. (2009). Biodiesel production from palm oil via heterogeneous transesterification. Biomass and bioenergy, 33(2), 271-276.
View at Publisher | View at Google Scholar - 41. Kansedo, J., Lee, K. T., & Bhatia, S. (2009). Cerbera odollam (sea mango) oil as a promising non-edible feedstock for biodiesel production. Fuel, 88(6), 1148-1150.
View at Publisher | View at Google Scholar - 42. Karmakar, A., Karmakar, S., & Mukherjee, S. (2010). Properties of various plants and animals feedstocks for biodiesel production. Bioresource technology, 101(19), 7201-7210.
View at Publisher | View at Google Scholar - 43. Kouzu, M., Yamanaka, S. Y., Hidaka, J. S., & Tsunomori, M. (2009). Heterogeneous catalysis of calcium oxide used for transesterification of soybean oil with refluxing methanol. Applied Catalysis A: General, 355(1-2), 94-99.
View at Publisher | View at Google Scholar - 44. Kraai, G. N., Schuur, B., Van Zwol, F., Van de Bovenkamp, H. H., & Heeres, H. J. (2009). Novel highly integrated biodiesel production technology in a centrifugal contactor separator device. Chemical Engineering Journal, 154(1-3), 384-389.
View at Publisher | View at Google Scholar - 45. Kumar, N., & Chauhan, S. R. (2013). Performance and emission characteristics of biodiesel from different origins: A review. Renewable and Sustainable Energy Reviews, 21, 633-658.
View at Publisher | View at Google Scholar - 46. Leung, D.Y.C., Guo, Y., (2006). Transesterification of neat and used frying oil: optimization for biodiesel production. Fuel Process. Technol. 87, 883–890
View at Publisher | View at Google Scholar - 47. Liang, S., Xu, M., & Zhang, T. (2013). Life cycle assessment of biodiesel production in China. Bioresource technology, 129, 72-77.
View at Publisher | View at Google Scholar - 48. Ling, J., Nip, S., Cheok, W. L., de Toledo, R. A., & Shim, H. (2014). Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresource Technology, 173, 132-139.
View at Publisher | View at Google Scholar - 49. Liu, X., He, H., Wang, Y., Zhu, S., & Piao, X. (2008). Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel, 87(2), 216-221.
View at Publisher | View at Google Scholar - 50. Ma, Y., Wang, Q., Zheng, L., Gao, Z., Wang, Q., & Ma, Y. (2016). Mixed methanol/ethanol on transesterification of waste cooking oil using Mg/Al hydrotalcite catalyst. Energy, 107, 523-531.
View at Publisher | View at Google Scholar - 51. Malins, K., Brinks, J., Kampars, V., & Malina, I. (2016). Esterification of rapeseed oil fatty acids using a carbon-based heterogeneous acid catalyst derived from cellulose. Applied Catalysis A: General, 519, 99-106.
View at Publisher | View at Google Scholar - 52. Marín-Suárez, M., Méndez-Mateos, D., Guadix, A., & Guadix, E. M. (2019). Reuse of immobilized lipases in the transesterification of waste fish oil for the production of biodiesel. Renewable Energy, 140, 1-8.
View at Publisher | View at Google Scholar - 53. Mathew, G. M., Raina, D., Narisetty, V., Kumar, V., Saran, S., Pugazhendi, A., & Binod, P. (2021). Recent advances in biodiesel production: Challenges and solutions. Science of the Total Environment, 794, 148751
View at Publisher | View at Google Scholar - 54. Mathiyazhagan, M., & Ganapathi, A. (2011). Factors affecting biodiesel production. Research in plant Biology, 1(2).
View at Publisher | View at Google Scholar - 55. Miao, X., Li, R., & Yao, H. (2009). Effective acid-catalyzed transesterification for biodiesel production. Energy Conversion and Management, 50(10), 2680-2684.
View at Publisher | View at Google Scholar - 56. Mizik, T., & Gyarmati, G. (2021). Economic and sustainability of biodiesel production—a systematic literature review. Clean Technologies, 3(1), 19-36.
View at Publisher | View at Google Scholar - 57. Nautiyal, P., Subramanian, K. A., & Dastidar, M. G. (2014). Production and characterization of biodiesel from algae. Fuel Processing Technology, 120, 79-88.
View at Publisher | View at Google Scholar - 58. Nakajima, K., & Hara, M. (2012). Amorphous carbon with SO3H groups as a solid Brønsted acid catalyst. ACS catalysis, 2(7), 1296-1304.
View at Publisher | View at Google Scholar - 59. Navajas, A., Campo, I., Moral, A., Echave, J., Sanz, O., Montes, M., ... & Gandía, L. M. (2018). Outstanding performance of rehydrated Mg-Al hydrotalcites as heterogeneous methanolysis catalysts for the synthesis of biodiesel. Fuel, 211, 173-181.
View at Publisher | View at Google Scholar - 60. OECD, I. (2016). Energy and air pollution: world energy outlook special report 2016.
View at Publisher | View at Google Scholar - 61. Press, E. (2015). C/10/L. 4 A/6/L. 4-Iinternational Renewable Energy Agency-Proposed Work Programme and Budget for 2016-2017.
View at Publisher | View at Google Scholar - 62. Paswan, D. (2021). Evaluation of thermal decomposition characteristics and kinetic parameters of melina wood. Biofuels, 13(1), 117-123.
View at Publisher | View at Google Scholar - 63. Prasad, S., Singh, A., Korres, N. E., Rathore, D., Sevda, S., & Pant, D. (2020). Sustainable utilization of crop residues for energy generation: A life cycle assessment (LCA) perspective. Bioresource Technology, 303, 122964.
View at Publisher | View at Google Scholar - 64. Rashid, U., & Anwar, F. (2008). Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil. Fuel, 87(3), 265-273.
View at Publisher | View at Google Scholar - 65. Rathore, V., Tyagi, S., Newalkar, B., & Badoni, R. P. (2015). Jatropha and Karanja oil derived DMC–biodiesel synthesis: A kinetics study. Fuel, 140, 597-608.
View at Publisher | View at Google Scholar - 66. Robert, V, Ramírez-Castrillón, M., Jaramillo-Garcia, V.P., Rosa, P.D., Landell, M.F., Vu, D., Fabricio, M.F., Ayub, M.A. Henriques, J.A. and Valente, P., (2017). The oleaginous yeast Meyerozyma guilliermondii BI281A as a new potential biodiesel feedstock: Selection and lipid production optimization. Frontiers in microbiology, 8, p.1776.
View at Publisher | View at Google Scholar - 67. Riazi, M. R., Pereira, R. G., Tulcan, O. E. P., Fellows, C. E., & Chiaramonti, D. (2017). Engine performance: biofuels versus petrofuels. In Biofuels Production and Processing Technology (pp. 569-586). CRC Press.
View at Publisher | View at Google Scholar - 68. Rincón, L. E.,Jaramillo, J. J. & Cardona, C. A. (2014). Comparison of feedstocks and technologies for biodiesel production: An environmental and techno-economic evaluation. Renewable Energy, 69, 479-487.
View at Publisher | View at Google Scholar - 69. Sadaf, S, Shahzadi, I., Iqbal, J., Ullah, I., & Bhatti, H. N. (2018). Evaluation of mustard oil for the synthesis of biodiesel: Pretreatment and optimization study. Environmental Progress & Sustainable Energy, 37(5), 1829-1835.
View at Publisher | View at Google Scholar - 70. Sieminski, A., Administrator, U. (2016). Energy information administration. International Energy Outlook.
View at Publisher | View at Google Scholar - 71. Silva, G. F., Camargo, F. L., & Ferreira, A. L. (2011). Application of response surface methodology for optimization of biodiesel production by transesterification of soybean oil with ethanol. Fuel Processing Technology, 92(3), 407-413.
View at Publisher | View at Google Scholar - 72. Sun, H., Ding, Y., Duan, J., Zhang, Q., Wang, Z., Lou, H., & Zheng, X. (2010). Transesterification of sunflower oil to biodiesel on ZrO2 supported La2O3 catalyst. Bioresource Technology, 101(3), 953-958.
View at Publisher | View at Google Scholar - 73. Sebitso, T., Kharidzha, M., & Harding, K. G. (2015). Life Cycle Assessment of biodiesel. Chemical Technology, 6.
View at Publisher | View at Google Scholar - 74. Sulaiman, S.& Talha, N. S, (2016). Overview of catalysts in biodiesel production. ARPN Journal of Engineering and Applied Sciences, 11(1), 439-442.
View at Publisher | View at Google Scholar - 75. Tabatabaei, M., Aghbashlo, M., Dehhaghi, M., Panahi, H. K. S., Mollahosseini, A., Hosseini, M., & Soufiyan, M. M. (2019). Reactor technologies for biodiesel production and processing: A review. Progress in Energy and Combustion Science, 74, 239-303.
View at Publisher | View at Google Scholar - 76. Thamsiriroj, T., & Murphy, J. D. (2011). A critical review of the applicability of biodiesel and grass biomethane as biofuels to satisfy both biofuel targets and sustainability criteria. Applied Energy, 88(4), 1008-1019.
View at Publisher | View at Google Scholar - 77. Umar, J. B, Babakura, M., Yelwa, J. M., Ibrahim, A., Aliyu, B. M., Yahaya, J. Y., & Ogabidu, A. O. (2019). Synthesis and characterization of biodiesel from Khaya senegalensis seed oil using heterogeneous catalyst. International Journal of Science and Management Studies (IJSMS), 2(4), 101-107.
View at Publisher | View at Google Scholar - 78. Vares, M., Sarmasti Emami, M. R., & Tahvildari, K. (2014). A novel membrane reactor for production of high-purity biodiesel. European Online Journal of Natural and Social Sciences, 3(3 (s)), pp-421.
View at Publisher | View at Google Scholar - 79. Wei, Z., Xu, C., & Li, B. (2009). Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresource technology, 100(11), 2883-2885.
View at Publisher | View at Google Scholar - 80. Xie, W., Yang, Z., & Chun, H. (2007). Catalytic properties of lithium-doped ZnO catalysts used for biodiesel preparations. Industrial & Engineering Chemistry Research, 46(24), 7942-7949.
View at Publisher | View at Google Scholar - 81. Yaqoob, H., Teoh, Y. H., Jamil, M. A., Rasheed, T., & Sher, F. (2020). An experimental investigation on tribological behaviour of tire-derived pyrolysis oil blended with biodiesel fuel. Sustainability, 12(23), 9975.
View at Publisher | View at Google Scholar - 82. Zeng, D., Li, R., Wang, B., Xu, J., & Fang, T. (2014). A review of transesterification from low-grade feedstocks for biodiesel production with supercritical methanol. Russian Journal of Applied Chemistry, 87, 1176-1183.
View at Publisher | View at Google Scholar - 83. Zhang, Y., Dubé, M. A., McLean, D. D., & Kates, M. (2003). Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresource technology, 90(3), 229-240.
View at Publisher | View at Google Scholar - 84. Zulqarnain, Mohd Yusoff, M. H., Ayoub, M., Ramzan, N., Nazir, M. H., Zahid, I., … & Butt, T. A. (2021). Overview of Feedstocks for Sustainable Biodiesel Production and Implementation of the Biodiesel Program in Pakistan. ACS omega, 6(29), 19099-19114.
View at Publisher | View at Google Scholar

 Clinic
Clinic
