Research Article | DOI: https://doi.org/10.31579/2834-5177/005
Long-Term follow up of HBV DNA Negative Patients with Chronic Hepatitis C Virus Treated with Direct Acting Antiviral Therapy for Hepatitis B Reactivation
- Enas M Kamal 1*
Enas Kamal, MD. Department of Gastroenterology, Hepatology and Endemic Medicine, Faculty of Medicine, Minia University, Address: 22-Elgeish street, Minia, Egypt.
*Corresponding Author: Enas Kamal, MD. Department of Gastroenterology, Hepatology and Endemic Medicine, Faculty of Medicine, Minia University, Address: 22-Elgeish street, Minia, Egypt.
Citation: Enas M Kamal, Yasser Fouad, Wael Abdel-Ghany, Yehia Sameh, Dina Attia. et all (2022). Long-Term follow up of HBV DNA Negative Patients with Chronic Hepatitis C Virus Treated with Direct Acting Antiviral Therapy for Hepatitis B Reactivation. International Journal of Clinical Infectious Diseases, 1(1) DOI:10.31579/2834-5177/005
Copyright: © 2022 Enas Kamal, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 08 September 2022 | Accepted: 15 August 2022 | Published: 21 September 2022
Keywords: HCV, hepatitis C virus; HBV; coinfection; reactivation, direct-acting antivirals
Abstract
Background: chronic HCV patients treated with direct-acting antivirals carry the risk of hepatitis B virus (HBV) reactivation in co-infected patients.
The aim was to estimate the risk of HBV reactivation in patients with negative HBVDNA for either HBsAg, anti-HBc or both, treated with direct-acting antivirals for chronic HCV infection.
Methods: 401 (3.8%) out of 10570 chronic hepatitis C patients were coinfected with HBV. In total 229 patients with negative HBV DNA were included in the study. Of them 85 patients (27.3%) were positive for both HBsAg and anti-HBc and 144 patients (45.7%) were positive for anti-HBc. All included patients received sofosbuvir (400mg)/ledipasvir (90mg) single oral dose for 12 weeks according to guidelines. HBsAg and HBV-DNA were monthly monitored for all patients in both groups throughout the study, until SVR12, then every three months till 48 weeks after end of treatment.
Results: Overall response was 96.5% with no significant difference in the 2 groups (p=0.982). In the HBsAg and anti-HBc group, two out of 85 patients (2.4%) showed HBV DNA >1log10 at 2nd and 3rd months post-SVR12, while only one patient out of 144 (0.7%) in the anti-HBc group seroreverted to HBsAg and HBV DNA >1log10 at the 3rd month after the end of treatment. In all 3 patients ALT showed rerise > 2 folds above the ULN. Fatigue was the only significant symptom by the reactivated patients. Conclusion: In HBV DNA-negative patients under DAAs the risk of reactivation is very low, however, long-term screening with HBsAg and ALT remains essential.
Introduction
Chronic liver disease secondary to dual hepatitis B and C coinfection is more severe and run an aggressive rapid course towards liver cirrhosis and hepatocellular carcinoma than each virus alone [1-3]. The principal role in the dual infection is played by the host immune response, causing predominance of either one of them [3]. In coinfection, HCV dominate due to the induction of interferons types I and III inhibiting HBV replication through inhibiting NS2 and NS5A core proteins [4]. Reactivation of suppressed HBV was faced in the HCV interferon-based treatment being based on the hepatitis C viral replication suppression with consequently reducing inflammation and fibrosis [5, 6]. The introduction of the direct-acting antivirals (DAAs) have largely suppressed HCV infection with excellent cure rate [7]. The reactivation of HBV in the era of DAAs are associated with considerable clinical attention [(8]. DAAs result in HBV replication and protein expression in coinfected patients [9]. The timing of reactivation might be early during the DAAs treatment [10]. Reactivation may be detected in the form of rise HBV DNA 1 to 2 log IU/mL or seroconversion of hepatitis B surface antigen (HBsAg) in negative patients with positive hepatitis B core antibody (anti-HBc) may also occur [11]. Another clinical consideration is the presence of anti-HBc with HCV which may also be associated with progressive liver disease [12]. The launch of HCV eradication campaign in Egypt was associated with DAAs treatment of over million and half of chronic HCV infected individuals. the risk of reactivation in genotype-4 chronic HCV infected patients under DAAs treatment, either coinfected or have anti-HBc, was not until now evaluated [13]. Therefore, the aim of this prospective study was designed to estimate the risk of hepatitis B reactivation in patients with HBV DNA negative and positive for either HBsAg, anti-HBc or both treated with direct-acting antivirals for chronic HCV infection.
Patients and Methods
Study plan:
This is a multicentre, prospective, study, where patients were recruited from multiple medical centres: Minia University hospitals, Beni-Suef University hospitals, Ain-Shams University hospitals, Egyptian Military Academy, and private medical centres. The study started simultaneously at the Virology unites of the diverse centres between January 2016 and January 2019. The study was performed in consistence with the Helsinki Declaration, with endorsement from the local Ethical Committee of each centre. A written informed consent was taken from all patients.
Study cohort:
A total of 10570 chronic hepatitis C patients from the Attendees of the Virology unites were screened for HBV coinfection by HBsAg and anti-HBc, then HBVDNA was done for positive patients for one or both markers. Two hundred and twenty-nine patients were HBV DNA negative, of them 85 patients (27.3%) were positive for both HBsAg and anti-HBc and 144 patients (45.7%) were positive anti-HBc and negative for both HBsAg. All included patients received one tablets containing 400 mg Sofosbuvir and 90 mg of ledipasvir once daily for 12 weeks. Patients enrolment described in (figure 1).
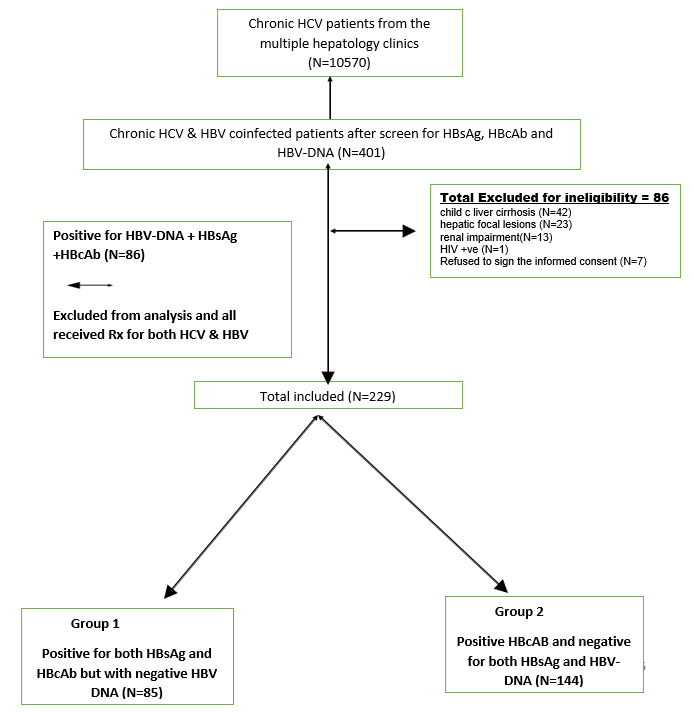
Abbreviations: HBV; hepatitis B virus, HBsAg; hepatitis B virus surface antigen, anti-HBc; hepatitis B virus core antibody, HCV; hepatitis C virus, HCC; hepatocellular carcinoma, HIV; human immunocompromised virus
Figure 1: flow diagram describing patients’ recruitment.
Virologic evaluation:
All patients were positive for HCV antibodies by the third generation enzyme linked immunosorbent assay (Orth. HCV 3.0 Orth., Raritan, NJ, USA) with infection confirmed by polymerase chain reaction and was quantitated in all patients using the amplicor HCV monitor assay (Roche, Diagnostic Systems, Branchburg, NJ, USA) The measurements were performed before starting the treatment, week 12 (EOT), and 12 weeks after end of treatment (SVR12). ALL patients were initially screened for HBsAg, and antibody against hepatitis B core antigen (anti-HBc) immunoglobulin G (IgG). If HBsAg and/or anti-HBc IgG were positive quantitative measurement of the viral load (VL) (quantitative HBV-DNA) and hepatitis B envelope antigen (HBeAg), and antibody to the envelope antigen (anti-HBe) were performed. DNA was extracted from the serum by QIAGEN viral DNA extraction kit (Qiagen GmbH, Hilden, Germany) using 200 ml of the patient serum following the manufacturer’s instructions.
Genotyping:
HCV genotyping was performed by direct sequencing of the 5’ untranslated region (5’UTR) using a RT-PCR-based assay (AmpliSens HCV-genotype-FRT PCR kit) for the HCV antibodies positive before treatment start. HBV genotyping was performed by Commercial direct sequencing assay kit (TRUGENE HBV Genotyping Kit; Siemens Medical Solutions Diagnostics, NY, USA). NS5A and NS5B resistance associated substitution (RAS) were evaluated using deep sequencing at least 15% cut off. Virological HBV reactivation was defined as de novo appearance of HBV DNA > 1-2 log10 in HBV DNA negative patients or seroreversion of HBsAg to positive in HBsAg-negative patients [11].
Assessment of liver fibrosis:
Staging of liver fibrosis was performed by transient Elastography (TE) (Fibroscan®, Echosens, France) Staging of liver fibrosis was evaluated into (kPa) according to the Metavir scoring system for chronic HCV [14].
Routine laboratory evaluation:
All cases in the studied groups (N=229) were followed thoroughly by liver function tests (alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, and bilirubin, international normalized ratio (INR), complete blood picture, were measured at baseline, weeks 4, 8, and 12 (End of treatment), and 12 weeks after end of treatment (SVR12), HBsAg, HBV DNA were monitored every 3 month until 48 weeks after the end of treatment.
Statistical analysis:
Categorical variables were summarized by number and percentages. Continuous variables were summarized by means and standard deviations or median and ranges. Mean percent of response of all patients was expressed as proportions and percentages (with upper and lower 95% confidence limits). Mean percent of HBV DNA reactivation was expressed as proportions and percentages. The Mann-Whitney test was used for comparisons between two independent variables. Any p-value lower than 0.05 was considered significant.
Results:
Qualities of the study accomplice:
In total, 229 chronic HCV patients with HBV DNA negative where included in this study, a group with HBsAg and anti-HBc positive (N= 85) and a group with anti-HBc positive and negative HBsAg (N= 144). The prevalence of HCV / HBV coinfected patients was (401/10570) (3.8%). The main of mode of HCV / HBV transmission was the positive family history 64/ 229 patients (27.9%) with at least one member in the family infected, then dental procedures were reported in 51 /229 patients (22.3%), while no aetiology was reported in 70/229 patients (30.6%). Patient’s characteristics are listed in (table 1).
Units Normal range | HBsAg positive† N=85 | HBsAg negative† N=144 | |
| Age | Mean ± SD (years) | 50±7.4 | 48±8.7 |
| Male gender | N/% | 57 (67) | 92 (64) |
| Mode of transmission | N/% |
|
|
| Family history of HCV |
| 27 (32%) | 37 (25.7%) |
| Dental operations |
| 20 (23.6%) | 31 (21.5%) |
| Surgical operations |
| 8 (9%) | 19 (13.2%) |
| Blood transfusion |
| 5 (6%) | 9 (6.3%) |
| Intravenous drug use |
| 2 (2.4%) | 1 (0.7%) |
| Unknown |
| 23 (27%) | 47 (32.6%) |
| Prior treatment | N/% |
|
|
| Naïve |
| 85 (100%) | 99 (69%) |
| IFNRIB |
| - | 7 (5%) |
| SOFRIB |
| - | 10 (7%) |
| SOFDCV |
| - | 13 (9%) |
| SOFDCVRIB |
| - | 8 (5%) |
| SOFSIM |
| - | 7 (5%) |
| Laboratory parameters |
|
|
|
| Hemoglobin | g/dL | 15.4±1.3 | 13.7±0.56 |
| TLC | 2.0-7.0 x10³/µL | 5.3±0.57 | 4.9±0.7 |
| Platelet count | (150-450 x103/µL) | 142.6±24 | 160±86 |
| INR |
| 1.10±1.0 | 0.99±0.84 |
| AST | (<30> | 83±27 | 82±25 |
| ALT | (<31> | 99±33 | 106±33 |
| Bilirubin | (1.2 mg/dL) | 0.83±0.09 | 0.84±0.09 |
| Albumin | (3.5-5 g/dL) | 3.8±0.4 | 4.2±0.4 |
| Creatinine | (0.6-1.2 mg/dL) | 0.84±0.11 | 0.85±0.11 |
| HCVRNA log10 | (IU/mL) | 6.0±2.4 | 5.6±2.6 |
| AFP | <11> | 7.1±9.0 | 5.9±5.9 |
Transient elastography* |
|
|
|
Absolute | kPa | 12±4.4 | 12.9±4.7 |
| 4 (5%) | 14 (10%) | |
F2 | 7.1 -9.9 kPa | 21 (25%) | 36 (25%) |
F3 | 10-12.9 kPa | 35 (41%) | 81 (56%) |
F4 | ≥ 13 kPa | 25 (29%) | 13 (9%) |
Abbreviations: HBsAg; hepatitis B virus surface antigen, anti-HBc; hepatitis B virus core antibody, IFNRIB; interferon / ribavirin, SOFRIB; sofosbuvir /ribavirin, SOFDCV, sofosbuvir / daclatasvir; SOFDCV/RIB, sofosbuvir /daclastavir / ribavirin; SOF/SIM, sofosbuvir/ simiprevir TLC; total leucocytic count, AST; aspartate aminotransferase, ALT; alanine aminotransferase, INR; international normalization ratio, g/dL, F; fibrosis stage, µL; microliter, IU; international unit, mg/dL; milligram/deciliter; ng/dL, nanogram/deciliter, N; number. *Liver fibrosis staging was diagnosed according the Metavir scoring system for transient elastography measurement of HCV. †Both groups were anti-HBc and HBVDNA negative.
Table 1: Baseline characteristics of the study groups
Effectiveness of HCV treatment in the studied groups:
The overall SVR12 rate was 221/229 (96.5%) with (95% CI 93.9% – 98.7%). In the SVR12 in the HBsAg and anti-HBc positive group was 82/85 (96.5%) with (95% CI 92.3% - 100%), and 139/144 (96.5%) with (95% CI 93.3%-99.3%) in the HBsAg negative and anti-HBc positive group.
Relapsers to HCV treatment:
In the HBsAg and anti-HBc positive group, 3 patients relapsed to the sofosbuvir/ledipasvir combination regimen with a mean age (50±9.5 years), ALT (69±17 IU/L) and AST (71±12 IU/L) rerise after SVR12. Platelet count was (142±329 x103/mm) and AFP was (8.3±3.8 ng/mL) and TE was 12.8±3.3 kPa with 2 patients were F3 and one patient F4. All patients did not show any HBV DNA rise. In the HBsAg negative and anti-HBc positive group, 5 patients relapsed to the sofosbuvir/ledipasvir combination regimen with a mean age (48±8.8 years), ALT (107±33 IU/L) and AST (82±25 IU/L). Platelet count was (159±86 x103 /mm), AFP was (8±4 ng/mL) and TE was 13.1±4.3 kPa with one patient F2 and 3 patients F3 and one patient F4. All patients were tested for RAS and were scheduled for retreatment accordingly.
HBV reactivation:
In the HBsAg and anti-HBc positive group, two out of 85 patients (2.4%) reactivated for HBV with HBV DNA became positive > 1log10 (113000, 230000 IU/mL, respectively) 2 and 3 months after SVR12 respectively. Both patients were males, 69 and 71 years and had liver cirrhosis. Both experienced rerise of liver enzymes upon the 2nd and 3rd month after SVR12 > 2 folds ULN (ALT 45/30 IU/L and 47/30 IU/ L and AST 55/31 IU/L and 66/31 IU/ L). Also, serum albumin level declined (3.2 and 3.3 gm/dl), and serum alpha-fetoprotein showed rise (65/11 and 52/11 ng/ml) than before SVR12. The serum total bilirubin and INR were in the normal range. Both patients started Entecavir 1 mg. Both patients were compliant and responders to the HCV treatment regimens and experienced no side effects during treatment except fatigue in the weeks HBV DNA reappeared. In the HBsAg negative and anti-HBc positive group, only one patient out of 144 (0.7%) patient seroreverted to HBsAg positive with HBV DNA became positive > 1log10 (123000 IU/mL). The patient was male (65 years) and had liver cirrhosis. He experienced rerise of liver enzymes 3 months after the end of HCV treatment > 2 folds ULN (ALT 67/30 IU/L and AST 88/31 IU/L). Serum alpha-fetoprotein level raised (56/11ng/mL). Serum albumin, bilirubin and INR remained within the normal ranges as prior to the activation. The patient was compliant and responder to the HCV treatment and experienced no side effects during or after the treatment. He received Entecavir 1 mg. (table 2 and figure 2). All patients were HBeAg negative.
Units Normal range | HBsAg positive N=85 | HBsAg negative N=144 | |
| Number of patients | n/% | 2 (2.4%) | 1 (0.7%) |
| Age | Mean± SD (years) | 69.5 | 65 |
| AST | (<30> | 60.5 | 88 |
| ALT | (<31> | 46 | 67 |
| AFP | (<11> | 58.5 | 56 |
| HBV DNA | (IU/mL) | 171.500 | 123.000 |
| Transient elastography | ≥ 13 kPa | F4 | F4 |
Abbreviations: HBsAg; hepatitis B virus surface antigen, anti-HBc; hepatitis B virus core antibody, AST; aspartate aminotransferase, ALT; alanine aminotransferase, AFP; alpha-fetoprotein, IU; international unit, ng/dL; nanogram/deciliter, N; number. Both groups were anti-HBc and HBVDNA negative.
Table 2: Characteristics of HBV reactivated patients in the study groups
No liver cirrhosis N=191 | Liver cirrhosis N=38 | |
| Age mean ± SD (years) | 48±8 | 54±7 |
| Child score | - | 5.5±0.5 |
| Platelet count x mm3/µL | 162±74 | 111±99 |
| AFP ng/mL | 4.8±3.6 | 15.4±12.8 |
| FIB-4 | 2.36±0.69 | 4.99±0.94 |
| TE kPa | 11.8±4.4 | 16.5±3.5 |
| Prior treatment |
|
|
| Naïve | 150 (79) | 34 (90) |
| IFNRIB | 7 (4) | - |
| SOFRIB | 10 (5) | - |
| SOFDCV | 13 (7) | - |
| SOFDCVRIB | 8 (4) | - |
| SOFSIM | 3 (1) | 4 (10) |
| Fibrosis stage |
|
|
18 (9) | - | |
| F2 | 57 (30) | - |
| F3 | 116 (61) | - |
| HBsAg+ve /HBsAg-ve | 60 (31) / 131 (69) | 25 (66) /13 (34) |
| HBV reactivation | 0 | 3 |
Abbreviations: HBV; hepatitis B virus, HBsAg; hepatitis B virus surface antigen, TE; transient elastography, kPa; kilopascal, IFNRIB; interferon / ribavirin, SOFRIB; sofosbuvir /ribavirin, SOFDCV, sofosbuvir / daclatasvir; SOFDCV/RIB, sofosbuvir /daclastavir / ribavirin; SOF/SIM, sofosbuvir/ simiprevir, µL; microliter, F; fibrosis, AFP; alfa-fetoprotein.
Table 3: Baseline characteristics and HBV reactivation in the cirrhotic versus the non-cirrhotic patients
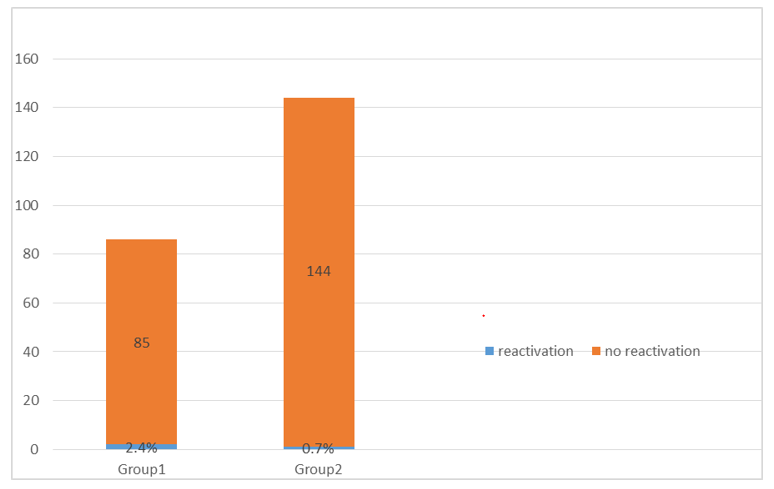
Figure 2: The prevalence of reactivation of Hepatitis B in the studied groups.
Discussion:
The national program launched in Egypt to eradicate HCV by 2020 with the use of DAAs allowed the screening for the HCV/HBV coinfection (15). In this large This study estimated the prevalence of HCV/HBV coinfection 401/10750 (3.8%). This study also estimated the risk of reactivation in chronic HCV patients treated with DAAs 3/229 (1.3%) in HBV DNA negative patients. The major consequence of HBV/HCV coinfection lies in rapid and aggressive progression of chronic liver disease and development of liver cirrhosis and hepatocellular carcinoma. Therefore, the importance of their identification and treatment rapidly plays an important role in order to avoid further progression of liver disease (16-18). This study also showed that follow up of cured HCV patients identified the reactivated HBV patients. Similarly, the response to the DAAs in HCV-HBV patients coinfected patients was equally effective and safe as in the non-coinfected patients.
Our prospective evaluation of this large cohort with chronic HCV infected DAAs-treated patients revealed that dual infection lies in the lower prevalence rate when compared with the worldwide distribution (1-15%) (Pol S, 2017 APT){Pol, 2017 #1} and the HBV DNA negative prevalence was 2.16% with the HBsAg carriers was 0.8%, which is lies in the minimal range in comparison to the worldwide distribution (2-10%) in those chronic HCV circulating HBsAg (Chakravati, A, Gastroenterol 2005). This study also revealed that the reactivation in the HBV DNA-negative patients with anti-HBc positive was either in the HBsAg positive (2.4%) or HBsAg negative (0.7%) was also small. This is consistent with a large national US cohort that included more than 17000 chronic HCV DAAs treated patients and found that the HBV reactivation was rare (3%) in non-reactive patients (19). Our study is also in line with the study performed by De Monte and his colleagues that stated that HBV reactivation was observed in one chronic HCV infected patient treated with sofosbuvir/ledipasvir combination who had resolved HBV infection(20). The results of our study in the non-reactive group is similar to several other studies (Sulkowski{S{Belperio, 2017 #2}{Londono, 2017 #16}{Yeh, 2015 #18}{Mucke, 2019 #20}ulkowski, 2016 #22}{Doi, 2017 #17}, Doi, Belpero, Londono, Mucke, Yeh, Wang), that found reactivation is minimal (0-2%) in that group of patients. In the active group of patients, our results were in line with 2 studies performed by Belperio and his colleagues and Serper and his colleagues (2%), in which their studies had proper sample size of the HBsAg positive patients {Belperio, 2017 #2}{Serper, 2018 #3}(Belperio, serper). However, other several studies, who included very small size of those group of patients, revealed higher percentage of reactivation ({Macera, 2017 #4}{Liu, 2018 #27}{Doi, 2017 #17}{Yeh, 2015 #18}{Londono, 2017 #16}Macera, Liu, Doi, Londono, Mucke, Yeh and Wang). Similarly, the meta-analysis by Jiang et al included 39 studies of previously treated HBV patients, HBV reactivation was observed in patients 21 % with DAAs vs 11% of those treated with interferon-based regimen. However, this meta-analysis included data from the previously mentioned studies of very small number of patients {Jiang, 2018 #19}(Jiang et al 2018) [21].
Although most if not all anti–HBc-positive individuals have replication-competent HBV DNA in the liver, the risk of reactivation is much lower in those who are HBsAg negative. This difference has been clearly shown in the setting of immunosuppression, with reactivation occurring in HBsAg-negative individuals only with profound immunosuppression (eg, rituximab, stem cell transplantation) and extremely rarely with lesser immunosuppression (eg, solid tumor chemotherapy, steroids) [27].
In our study the timing of HBV reactivation after DAAs was delayed, the earliest was 3 months after the end of treatment and the other 2 reactivation were 2 and 3 months after SVR12 which is similar to one study which showed HBV reactivation was detected one year after DAAs treatment [22]. However, other studies showed an earlier reactivation showed earlier reactivation in the first 2nd and 3rd month of DAAs treatment in HBsAg patients {Macera, 2017 #4} (Macera et al., Clin Gastroenterol Hepatol 2017).
The response of the sofosbuvir/ledipasvir combination in the HCV-HBV coinfected patients was similar in the non-coinfected patients(28, 29) and similar to the previously published data (20, 30)20, 29. Also, the side effects were minimal with no reported serious side effects. The reactivated patients complained from fatigue which was correlated with the inflammatory activity and HBV DNA appearance. The reactivation was also detected after end of treatment in one patient with anti-HBc positive HBsAg negative and after SVR12 in 2 patients with HBsAg and anti-HBc positive. This means that regular follow up of co-infected patients after SVR12 at least with liver enzymes is important to lower the cost, and in case of liver enzymes elevation HBsAg and HBV DNA must be considered. However, the presentation of reactivation was not in line with the other studies revealing acute hepatitis, need for liver transplantation or even death {Bersoff-Matcha, 2017 #9}Bersoff-Matcha SJ, Ann Intern Med. 2017,). A meta-analysis conducted by Chen et al revealed no difference in the incidence of HBV reactivation between the interferon-based treatment and DAAs based treatment, however, it occurred earlier, and the hepatitis presentation were more common in the DAAs-based co-infected patients (Chen et al., 2018)
In HCV/HBV co-infected patients, HBV inhibition is mediated by several HCV core proteins, mainly NS2 and NS5A and the potent suppression of DAAs to the HCV, that might consequently result in HBV replication and protein [removed]Shih CM, 1993, 1995 J Virol Chen SY, J Biol Chem. 2003;278:591–607... {Blackard, 2018 #11}{Chen, 2003 #14}Blackard and Sherman Rev Med Virol. 2018 July. Although, this reactivation was also observed with interferon-based treatment as well, however, the timing of the presentation was delayed, and this was implied by the role of interferon against HBV and clinical presentation was milder.
Limitations of this study included absent liver biopsy to evaluate the change of liver fibrosis. A second limitation was the short term follow up (only 1 year) of the patients, therefore, a longitudinal long-term follow-up is requested to estimate if a larger group of HBV reactivation will appear or decompensation in the cirrhotic group and the rate of progression in the non-reactivated co-infected patients. The strength of this study lies on being a real-world large cohort with HBV-negative patients with long-term follow up.
In conclusion, this study included large group of chronic HCV patients who were screened for occult and non-occult HBV with no previous HBV treatment and revealed a minimal risk of reactivation under DAAs treatment. However, baseline screening before treatment start is essential and follow up after SVR12 is recommended in all co-infected patients to ensure patients’ safety. The response to the DAAs in the co-infected patients is not different than the non-coinfected patients.
Conflict of Interest
We disclose any financial support or conflict of interest
Author’s contributions: Enas Kamal and Yasser Fouad, designed the concept of the study. Enas Kamal, Yasser M Fouad and Dina Attia wrote, analyzed and interpreted the data. All other authors collected the data. All authors reviewed the manuscript and approved the final version to be published. Dina Attia and Hisham Elkhayat contributed equally to be last authors.
List of Abbreviations:
HCV : hepatitis C virus
HBV : hepatitis B virus
EOT : end of treatment
SVR12 : Sustained virologic response at week 12
N : number
TE : transient elastography
Kpa : kilopascals
INR : international normalization ratio
AST : aspartate aminotransaminase
ALT : alanine aminotransferase
F : fibrosis
DAAs : directly acting antiviral drugs
HCC : Hepatocellular carcinoma
HBsAg : Hepatitis B surface antigen
Anti-HBc: Hepatitis B core antibodies
References
- Liu CJ, Liou JM, Chen DS, Chen PJ. Natural course and treatment of dual hepatitis B virus and hepatitis C virus infections. J Formos Med Assoc 2005;104:783-791.
View at Publisher | View at Google Scholar - Mekky MA, Nasr AM, Saleh MA, Wasif NK, Khalaf M, Aboalam H, Haredy M. Virologic and histologic characterisation of dual hepatitis B and C co-infection in Egyptian patients. Arab J Gastroenterol 2013;14:143-147.
View at Publisher | View at Google Scholar - Liaw YF, Chen YC, Sheen IS, Chien RN, Yeh CT, Chu CM. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. Gastroenterology 2004;126:1024-1029.
View at Publisher | View at Google Scholar - Wiegand SB, Jaroszewicz J, Potthoff A, Honer Zu Siederdissen C, Maasoumy B, Deterding K, Manns MP, et al. Dominance of hepatitis C virus (HCV) is associated with lower quantitative hepatitis B surface antigen and higher serum interferon-gamma-induced protein 10 levels in HBV/HCV-coinfected patients. Clin Microbiol Infect 2015;21:710 e711-719.
View at Publisher | View at Google Scholar - Chen DS. Fighting against viral hepatitis: lessons from Taiwan. Hepatology 2011;54:381-392.
View at Publisher | View at Google Scholar - Yan LB, Rao HY, Ma YJ, Bai L, Chen EQ, Du LY, Yang RF, et al. Hepatitis B virus infection in Chinese patients with hepatitis C virus infection: prevalence, clinical characteristics, viral interactions and host genotypes: a nationwide cross-sectional study. BMJ Open 2016;6:e012016.
View at Publisher | View at Google Scholar - Potthoff A, Berg T, Wedemeyer H, Group H-NBCCS. Late hepatitis B virus relapse in patients co-infected with hepatitis B virus and hepatitis C virus after antiviral treatment with pegylated interferon-a2b and ribavirin. Scand J Gastroenterol 2009;44:1487-1490.
View at Publisher | View at Google Scholar - Rodriguez-Inigo E, Bartolome J, Ortiz-Movilla N, Platero C, Lopez-Alcorocho JM, Pardo M, Castillo I, et al. Hepatitis C virus (HCV) and hepatitis B virus (HBV) can coinfect the same hepatocyte in the liver of patients with chronic HCV and occult HBV infection. J Virol 2005;79:15578-15581.
View at Publisher | View at Google Scholar - Pan Y, Wei W, Kang L, Wang Z, Fang J, Zhu Y, Wu J. NS5A protein of HCV enhances HBV replication and resistance to interferon response. Biochem Biophys Res Commun 2007;359:70-75.
View at Publisher | View at Google Scholar - Chen G, Wang C, Chen J, Ji D, Wang Y, Wu V, Karlberg J, et al. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: A systematic review and meta-analysis. Hepatology 2017;66:13-26.
View at Publisher | View at Google Scholar - Gonzalez SA, Perrillo RP. Hepatitis B Virus Reactivation in the Setting of Cancer Chemotherapy and Other Immunosuppressive Drug Therapy. Clin Infect Dis 2016;62 Suppl 4:S306-313.
View at Publisher | View at Google Scholar - De Maria N, Colantoni A, Friedlander L, Leandro G, Idilman R, Harig J, Van Thiel DH. The impact of previous HBV infection on the course of chronic hepatitis C. Am J Gastroenterol 2000;95:3529-3536.
View at Publisher | View at Google Scholar - Liu Z, Hou J. Hepatitis B virus (HBV) and hepatitis C virus (HCV) dual infection. Int J Med Sci 2006;3:57-62.
View at Publisher | View at Google Scholar - Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008;134:960-974.
View at Publisher | View at Google Scholar - El-Akel W, El-Sayed MH, El Kassas M, El-Serafy M, Khairy M, Elsaeed K, Kabil K, et al. National treatment programme of hepatitis C in Egypt: Hepatitis C virus model of care. J Viral Hepat 2017;24:262-267.
View at Publisher | View at Google Scholar - Konstantinou D, Deutsch M. The spectrum of HBV/HCV coinfection: epidemiology, clinical characteristics, viralinteractions and management. Ann Gastroenterol 2015;28:221-228.
View at Publisher | View at Google Scholar - Nguyen LH, Nguyen MH. Systematic review: Asian patients with chronic hepatitis C infection. Aliment Pharmacol Ther 2013;37:921-936.
View at Publisher | View at Google Scholar - Alberti A, Vario A, Ferrari A, Pistis R. Review article: chronic hepatitis C--natural history and cofactors. Aliment Pharmacol Ther 2005;22 Suppl 2:74-78.
View at Publisher | View at Google Scholar - Serper M, Forde KA, Kaplan DE. Rare clinically significant hepatic events and hepatitis B reactivation occur more frequently following rather than during direct-acting antiviral therapy for chronic hepatitis C: Data from a national US cohort. J Viral Hepat 2018;25:187-197.
View at Publisher | View at Google Scholar - De Monte A, Courjon J, Anty R, Cua E, Naqvi A, Mondain V, Cottalorda J, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: Reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol 2016;78:27-30.
View at Publisher | View at Google Scholar - Jiang XW, Ye JZ, Li YT, Li LJ. Hepatitis B reactivation in patients receiving direct-acting antiviral therapy or interferon-based therapy for hepatitis C: A systematic review and meta-analysis. World J Gastroenterol 2018;24:3181-3191.
View at Publisher | View at Google Scholar - Mucke MM, Mucke VT, Peiffer KH, Sarrazin C, Zeuzem S, Berger A, Vermehren J. Absence of HBV Reactivation in Patients With Resolved HBV Infection Following DAA Therapy for Hepatitis C: A 1-Year Follow-up Study. Open Forum Infect Dis 2019;6:ofy340.
View at Publisher | View at Google Scholar - Takayama H, Sato T, Ikeda F, Fujiki S. Reactivation of hepatitis B virus during interferon-free therapy with daclatasvir and asunaprevir in patient with hepatitis B virus/hepatitis C virus co-infection. Hepatol Res 2016;46:489-491.
View at Publisher | View at Google Scholar - Sulkowski MS, Chuang WL, Kao JH, Yang JC, Gao B, Brainard DM, Han KH, et al. No Evidence of Reactivation of Hepatitis B Virus Among Patients Treated With Ledipasvir-Sofosbuvir for Hepatitis C Virus Infection. Clin Infect Dis 2016;63:1202-1204.
View at Publisher | View at Google Scholar - Loggi E, Gitto S, Galli S, Minichiello M, Conti F, Grandini E, Scuteri A, et al. Hepatitis B virus reactivation among hepatitis C patients treated with direct-acting antiviral therapies in routine clinical practice. J Clin Virol 2017;93:66-70.
View at Publisher | View at Google Scholar - Londono MC, Lens S, Marino Z, Bonacci M, Ariza X, Broquetas T, Pla A, et al. Hepatitis B reactivation in patients with chronic hepatitis C undergoing anti-viral therapy with an interferon-free regimen. Aliment Pharmacol Ther 2017;45:1156-1161.
View at Publisher | View at Google Scholar - Paul S, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Hepatitis B Virus Reactivation and Prophylaxis During Solid Tumor Chemotherapy: A Systematic Review and Meta-analysis. Ann Intern Med 2016;164:30-40.
View at Publisher | View at Google Scholar - Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, Nakane K, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis 2015;15:645-653.
View at Publisher | View at Google Scholar - Bourliere M, Bronowicki JP, de Ledinghen V, Hezode C, Zoulim F, Mathurin P, Tran A, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis 2015;15:397-404.
View at Publisher | View at Google Scholar - Liu CJ, Chuang WL, Sheen IS, Wang HY, Chen CY, Tseng KC, Chang TT, et al. Efficacy of Ledipasvir and Sofosbuvir Treatment of HCV Infection in Patients Coinfected With HBV. Gastroenterology 2018;154:989-997.
View at Publisher | View at Google Scholar

 Clinic
Clinic
