Research Article | DOI: https://doi.org/10.31579/2834-5029/038
Investigation of Pittosporum Moluccanum Plant Extracts as Potential Source of Biopesticides Against Snails and Weeds
- Melissa June V. Paderog *
- Remi Charlene M. Salvilla
Pharmacy Department, College of Pharmacy and Medical Technology, University of San Agustin, Gen. Luna Street, Iloilo City, Philippines.
*Corresponding Author: Melissa June V. Paderog, Pharmacy Department, College of Pharmacy and Medical Technology, University of San Agustin, Gen. Luna Street, Iloilo City, Philippines.
Citation: Paderog MJV., Salvilla RCM., (2024), Investigation of Pittosporum Moluccanum Plant Extracts as Potential Source of Biopesticides Against Snails and Weeds, International Journal of Biomed Research. 3(1): DOI: 10.31579/2834-5029/038
Copyright: © 2024, Melissa June V. Paderog. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cite.
Received: 01 December 2023 | Accepted: 27 December 2023 | Published: 03 January 2024
Keywords: pittosporum molucannum; biopesticides; molluscicide; herbicide; natural product
Abstract
Pesticidal poisoning has been relatively uncontrolled since the 1960s. The rate of intentional and unintentional pesticide poisoning cases reflects how extensively the community and local agriculture uses these agents. Pesticides are mostly synthetic and toxic substances. Its health risks outweigh the benefits it possesses; thus, it is necessary to look for a natural and less toxic alternative. Pittosporum moluccanum was traditionally used as an herbal pesticide; however, no scientific findings support this claim. Thus, we aim to substantiate the plant’s pesticidal effect. Briefly, the ethanolic extracts of the leaves, fruits, and barks were obtained and chemically profiled using TLC principles. Then, their toxicity was screened using brine shrimp lethality assay and MTT cytotoxicity assay. The pesticidal activity of the extracts was determined using phototoxicity assay against barnyard seeds and immersion screening method against golden apple snails comparing their activities against glyphosate and metaldehyde as positive controls and, water and DMSO as growth controls. Chemically, the extracts contain semipolar and UV-active secondary metabolites that belong to the alkaloids, flavonoids, phenolics, and tannins group of compounds. Moreover, toxicity profiling suggests an LC50 of 0.098 mg/mL for leaves extract while 3.125 mg/mL for bark and fruit extracts; and, CC50 of 0.39 ug/mL for fruit extract while 0.2 ug/mL for both leaves and bark extracts. For pesticidal activity, seed germination inhibition of 85.56% (±1.20SD), 87.78% (±1.22SD), and 91.11% (±1.28SD) was observed on the leaves, fruits, and bark, respectively, while the positive control (1 µg/mL glyphosate) only exhibited 84.44% ± 1.57 SD. Immersion assay results suggest that the plant extracts exhibit a concentration-dependent molluscicidal activity against the snails and a 71.11% (±1.33SD) activity for 6 µg/mL metaldehyde (positive control). Our findings suggest that P. molucannum plant contains compounds that exhibit pesticidal activities against snails and weeds which are the most common pests in agricultural lands.
Introduction
Unintentional pesticide poisoning (UPP) in humans has been considered one of the inherent public health problems most developing and agricultural countries have faced since the 1960s. About 300 million cases of UPP with 11,000 fatalities yearly are reported cases, which could probably be much higher since many cases remain unreported and unrecorded because patients do not seek medical attention. Of these cases, 57.99 % were recorded instances from the Philippines [1]. Moreover, it was claimed that pesticides also accounted for about 20 % of global suicides leading to almost 16,000 fatalities annually from 2010-2014 [2]. Mixed pesticide poisoning is one of the leading causes of poisoning in the Philippines. It ranked sixth based on the total poisoning cases recorded in the country from 2004 to 2009 [3].
Pesticides are substances used to kill, repel, or control plants (herbicides) and animals (insecticides and rodenticides) considered pests. They help control the spread of insects, bacteria, fungi, and algae, not just in crops but also in refrigerators, paint, carpets, and food packaging materials [4]. Widespread agricultural and household use of these pesticides exposes people to low levels through their diets (food and water). Therefore, people who work directly (farmers and agriculturists) with these pesticides are at high risk for their adverse effects. A few examples of its harmful effects on humans are skin and eye irritations, asthma, allergies and hypersensitivity, nerve disorders, disorders of the immune and endocrine systems, and cancer [4-10].
As for their harmful impact on the environment, pesticides threaten species' biodiversity in terrestrial (e.g., birds and bees) and aquatic (e.g., species of salmon) environments [11]. Furthermore, the accumulation of these toxins in the environment indirectly decreases the prey in the food chain, thus compromising our ecosystem [12,13].
With this, several resolutions and programs were developed to reduce the harmful effects of pesticides on humans and the environment. Examples of these resolutions and programs are the (a) Montreal Protocol on Substances that Deplete the Ozone Layer, which is a global treaty controlling and phasing out the use of methyl bromide as it depletes our ozone layer, and (b) the Stockholm Convention on Persistent Organic Pollutants that prohibits and restricts the used of certain kinds of hazardous pesticide. However, the considerable challenge in implementing these resolutions and programs is the authorization and testing requirements for product registration of these pesticides since there is no intact protocol or guidelines for risk assessment, particularly chronic toxicity studies established for this matter. Moreover, with the demand for crops for export, farmers strongly rely their productivity on pesticides, hence aggravating human exposure to these toxins. Therefore, continued pesticide poisoning cases will still be observed without strict regulations and standardized protocols in these pesticides' product registry and pharmacovigilance monitoring [14]. In such matters, safer alternative approaches (e.g., the use of plant pesticides) coupled with sustainable agricultural practices are highly recommended to be used. New kinds of practical plant pesticides with potentially high activity but lower or no impact on humans and the environment are greatly needed [15].
The Pittosporum genus belongs to the family Pittosporaceae of the order Apiales that are naturally distributed in Australia and Asia (China, Japan, Indonesia, and the Philippines) [16-18]. Species belonging to this genus grow as shrubs or slender trees up to 30 m high and possess several biological activities. Methanolic extract of stem bark of Pittosporum mannii Hook was reported to have antimalarial activity against chloroquine-resistant strains with an IC50 of 4.3 μg/mL [19]; P. viridiflorum Sims is used traditionally for the management of fungal infections in HIV/AIDS patients, and its leaves were reported to possess antibacterial activity [20-22]; and molluscicidal activity was also reported on P. tobira varigatum [23]. Moreover, antimicrobial activity was also reported on P. angustifolium leaves and aqueous fruit extracts [24-26]; hepatoprotective effects were reported on the stem bark of P. neelgherrense [27]; antibacterial, antifungal, anti-inflammatory, analgesic, and anxiolytic activities were written on P. floribundum [28]. These biological activities are attributed to various bioactive compounds in this genus: saponins, flavonoids, terpenes, triterpenes and sesquiterpenes, phenols, polyphenols, tannins, glycosides, steroids, and volatile oils [21,22,26,28-30]. One notable species of Pittosporum found widespread in the Philippines is P. moluccanum, also known as P. timorense, and it was reported to be abundantly growing in Northwestern Luzon [19,20]. The plant has reported antioxidant activity by scavenging hydroxyl ions, which has an IC50 of 0.16-0.67 μg/mL [30], and antimalarial activity [18]. Ethnobotanical use of the plant suggests its traditional use as an herbal pesticide though no further scientific findings support this claim [31,32]. P. moluccanum leaves and fruits contain flavonoids, tannins, saponins, steroids, and alkaloids [33], and the chemical profile of other plant parts was limitedly explored. Moreover, no toxicity studies were done on the plant. However, mutagenicity was reported on P. pentandrum, and weak cytotoxicity against the A2780 human ovarian cancer cell line was reported on P. viridiflorum, which belongs to the same genus as P. moluccanum [25]. With the emerging cases of UPP, the harmful effects that synthetic pesticides produce, and the potential of the P. molucannum plant as a source of natural biopesticides, it is necessary that we look for natural options that are not harmful to consumers and the environment. Thus, this study was conceptualized. Generally, this study would like to investigate and provide initial findings on the biological activity of P. moluccanum as a natural biopesticide. Specifically, this study aimed to (a) chemically profile the plant through chromatographic technique; (b) evaluate the toxicity of the plant by determining its cytotoxicity concentration (CC50) on Saccharomyces cerevisiae as the test organism using MTT assay and lethal concentration (LC50) using brine shrimp lethality assay; and, (c) determine the plant's effectiveness as (i) molluscicide against golden apple snails (Pomacea canaliuculata) using the guidelines set by WHO for testing molluscicides, and (ii) herbicide against barnyard weed (Echinochloa crusgalli) seeds through phytotoxicity assay.
Materials and Methods
Materials
This study utilized reagent grade ethanol as extraction solvent, ethyl acetate and dichloromethane as thin layer chromatography mobile phase, Dragendorff reagent, aluminum chloride solution, ferric chloride solution, and Liebermann’s Buchard reagent as color-forming reagents in phytochemical profiling by TLC method. Artemia salina larvae were used for the lethality assay, and Saccharomyces cerevisiae ATCC 20784 was used for the cytotoxicity assay. Moreover, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) solution in phosphate buffer saline (PBS; 0.1 M; 7.4 pH) was used in the cytotoxicity assay. Golden apple snail (Pomacea canaliculata) was used as the test organism for molluscicidal activity determination, while seeds of barnyard grass (Echinochloa crusgalli) were used as the test organism in herbicidal activity determination. Furthermore, metaldehyde and glyphosate were used as positive controls for molluscicidal and herbicidal assays, respectively.
Sampling site and sample collection
The leaves, barks, and fruits of P. molucannum were collected in coordination with the Local Government of Sipalay and Latasan Beach Resort, where the plant was found abundantly growing. Specifically, the plant samples were collected at 9.735597 latitudes, 1.393956 longitudes, and 11.21 m altitude. Leaves, bark, and fruits were collected from randomly selected trees of P. moluccanum, placed in screen bags, and kept in storage containers with desiccants. The plant samples were brought to the Pharmacy Laboratory of the University of San Agustin for processing.
Sample preparation and extraction
The collected plant parts were cleaned, tapped dry, and placed in aluminum trays for oven drying at 60 0C until the constant dried weight was obtained. Dried samples were pulverized using a mechanical grinder, and powdered materials were macerated in enough volume of ethanol for 24 hrs. After maceration, the mixtures were filtered, and the filtrate was concentrated using a rotary evaporator (at 50 0C water bath). Extracts were then stored in a -20 0C freezer until further use [34-36].
Chemical profiling of the different plant parts
This test was done to chemically profile the different plant parts of P. molucannum using the thin-layer chromatography (TLC) technique. Briefly, the ethanolic extracts were spotted onto pre-coated TLC plates accordingly and eluted using an ethyl acetate – dichloromethane (1:13) solvent system to obtain a well-defined resolution of bands in the chromatogram. Phytochemical screening for the presence of different compounds was performed on chromatograms using color-forming reagents. Dragendorff reagent (NaNO2) was used to detect alkaloids [37,38], aluminum chloride (AlCl3) test was used to detect flavonoids [39], ferric chloride (FeCl3) test was used to detect tannins and phenolics [40-42], and terpenes were detected using Liebermann's Buchard (Vanillin sulfate test) [43].
CC50 and LC50 determination of extracts
This test was done to profile the toxicity of the plant initially. In this test, cytotoxicity (CC50) was determined through MTT assay using S. cerevisiae as the test organism. On another note, lethal concentration (LC50) was determined using brine shrimp (Artemia salina) lethality assay.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay
In this test, a 48-h culture of Saccharomyces cerevisiae ATCC 20784 with a cell density of 0.05 OD620nm in Saboraud Dextrose Broth (SDB) was prepared and used in the assay. Test inoculum (190 µL/well) was exposed to 10 μL of 2-fold serially diluted concentrations (dilution range 12.5 – 0.02 µg/mL) of the extracts for 48 hr at 35 0C. Untreated cells were also prepared for control. After incubation, the cells were harvested by centrifugation (3000 rpm for 1 min), and the pelleted cells were resuspended with 100 μL of phosphate buffer saline (0.1 M PBS – 7.4 pH). Then, MTT dye solution (5 mg/mL PBS) was added, and the mixtures were kept in the dark for three hours. Then the formazan crystals formed in the mixture were harvested and resuspended in DMSO. Absorbance values of the reaction mixtures were then read using a microplate reader at 570 nm wavelength [44-46]. Absorbance values were then analyzed where high absorbance values indicate high cellular activity and low values indicate low cellular activity (indicating dead cells). Percent toxicity was calculated using the equation below, and CC50 was determined as the lowest concentration exhibiting 50 % toxicity.

Brine shrimp lethality assay
In this test, brine shrimp larvae (Artemia salina) were utilized as the test organism. The larvae were grouped (10 larvae per group where 1 group is 1 replicate) and exposed to 2-fold serially diluted concentrations (dilution range of 6.25 – 0.012 mg/mL) of the extract in DMSO. Survival of the larvae was then recorded after 24 h treatment exposure. Percent (%) mortality and corresponding % lethality of each concentration was calculated using the equation below [47]. Lethal concentration (LC50) was determined as the lowest concentration that exhibits 50 % lethality in the larvae.
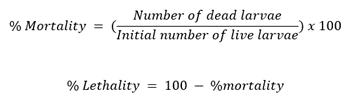
Pesticidal activity screening
This test was conducted to preliminary screen the potential pesticidal activity of the plant on snails and whitefly, which are the most common agricultural crop pests, and on Echinochloa crusgalli seedlings for their potential in weed control. The screening focuses explicitly on the plant's molluscicidal and herbicidal activity.
Molluscicidal activity determination (Immersion Bioassay)
This experiment was done following the WHO-set procedures using golden apple snails (Pomacea canaliculata) as the test organism to determine the effective concentration of plant extracts to kill 100 % of the snail population. The experiment utilized increased concentrations of the different plant extracts based on their respective CC50 (10x, 15x, 50x CC50, 250 µg/mL, and 500 µg/mL). DMSO and 6 µg/mL metaldehyde were used as the negative and positive control, respectively. Untreated snails were also prepared as a control. The snails were randomly grouped into 10 snails per concentration per trial. The snails were placed in 500 mL microwaveable tubs (covers were perforated) and submerged in 500 mL extract and control–mixed water. The tubs were covered, and the snails were exposed to the treatments for 24 h. After exposure, the snails were rinsed with water, transferred to natural snail water in a new container, and mortality was recorded after 24 h recovery period. Snails that will not move upon disturbance and remain in their shells will be considered dead [48,49]. Corrected percent mortality and its corresponding percent lethality were calculated using the equation below. The lowest concentration that kills all the snails (100 % lethality) was considered the lethal concentration of the extract. The test was performed in 3 trials.
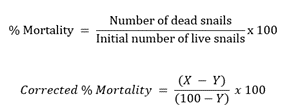
Where:
X = percent mortality of the treatment
Y = percent mortality of the untreated group
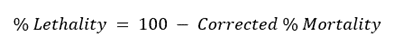
Herbicide activity determination (Phytotoxicity Assay)
This test was done to evaluate the herbicide activity of the extract on Echinochloa crusgalli seeds using phytotoxicity assay. The seeds were collected from a rice field where the plant is abundantly growing in Sipalay City, Negros Occidental. Ten times (10x) CC50 of the different plant extracts were evaluated in this experiment. The extract solution was prepared by dissolving the calculated cytotoxic concentration in ethanol, then Tween-20 (250 μg/mL) was added, and the addition of distilled water prepared a final volume of 5 mL. Then 3 mL of the prepared solution was transferred onto a 90 mm petri dish with Whatman filter paper. Then seeds were surfaced, sterilized with methanol for about 10 s, and washed with distilled water. The surface sterilization was done 3 times to remove any dirt and bacterial contamination. Then, 10 seeds were placed in the petri dish containing the treatment solution and were sealed with a parafilm. Elongation (length) of roots and shoots of the seedlings was evaluated after 5 days of cultivation at room temperature with a 16/8 h light/dark photoperiod. The number of seeds that have germinated was recorded, and % germination was calculated using the formula below [50-52]. The test will be done in three trials where tween-20 (250 μg/mL) in water (negative) and glyphosate (positive) were used as controls.
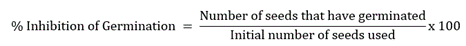
Waste disposal management
The apparatus and instruments used in the biological assays underwent chemical sterilization using 10% hypochlorite solution and physical method by steam sterilization using an autoclave for 30 min at 121 0C (15 psi), if applicable. Used reagents were classified and disposed of accordingly based on the waste disposal management protocol of the university. In addition, biological test organisms, namely snails and seedlings, were disposed of as biohazard wastes.
Results and Discussion
Extraction of the different plant parts of Pittosporum molucannum
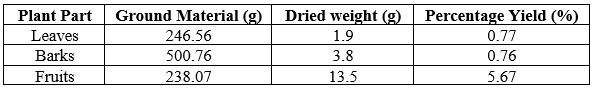
The plant samples were weighed, washed with tap water twice, finally rinsed with distilled water, and tapped dry with tissue paper. Samples were then placed in trays and oven dried at 60 0C until a constant weight was obtained. The different plant parts were ground using a mechanical grinder and macerated in enough ethanol for 24 h. After which, the macerated samples were filtered, and filtrates were concentrated in a vacuum using a rotary evaporator with a water bath set at 50 0C. The concentrated extracts were transferred onto their corresponding pre-weighed scintillation vial and continued to concentrate until total dryness. (Table 1).
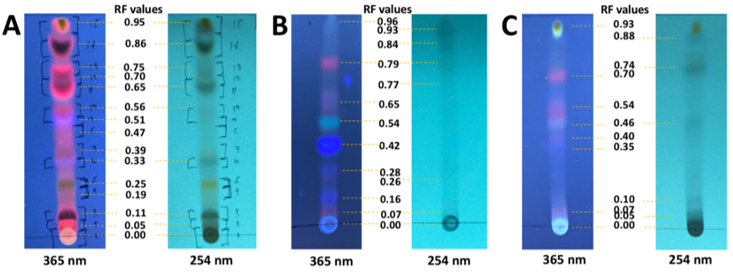
Phytochemical profiling of the different plant parts
This experiment was conducted to profile the compounds in the different plant extracts using chromatographic principles. Here, plant samples were spotted onto their corresponding TLC plates and eluted using ethyl acetate – dichloromethane (DCM) (1:13) solvent system. The developed chromatograms were visualized under UV 254 nm and UV 365 nm. Results of
the TLC experiment suggest that the extracts contain semipolar compounds. Specifically, the leaves have nine (9) bands active at UV 54 nm and 15 bands active at UV 365 nm. The barks contain seven (7) bands seen at UV 254 nm and nine (9) bands at UV 365 nm, while fruits contain six (6) bands at UV 254 nm and eight (8) bands at UV 365 nm. (Figure 1).
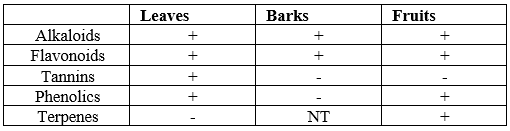
Moreover, TLC profiling using color-forming reagents was performed to identify the presence of secondary plant metabolites. Briefly, prepared chromatograms of the extracts were flooded with different reagents, and positive color changes were observed accordingly. Alkaloids were detected using Dragendorff reagent (NaNO2), flavonoids were detected using
Aluminum chloride (AlCl3), tannins and phenolics were detected using Ferric chloride (FeCl3), and terpenes were detected using Liebermann's Buchard (Vanillin sulfate test). Results showed that alkaloids and flavonoids were present in all three extracts, phenolics were only in leaves and fruits, and tannins were only in leaves (see Table 2).
Our natural resources are an abundant reservoir not just of food and nutrient but also of bioactive molecules from which most of our medicines are derived. The medicinal properties of these natural resources, particularly of plants, are generally dependent on the various phytochemicals they produce, like alkaloids, known anticancer agents, and flavonoids as antibacterial agents. Plants produce these secondary metabolites for survival, as protection from diseases, pollution, stress, and UV rays. These metabolites also contribute to their color, aroma, and flavor. Known diverse major classifications and important phytochemicals are alkaloids, flavonoids, phenolics, tannins, saponins, terpenes and steroids, glycosides, and terpenes. Phenolics are known to reduce oxidative stress, thus, target a wide array of health conditions, including cardiovascular diseases and cancer[53]; alkaloids are known to have hypoglycemic effects, antibacterial, and anti-apoptosis aside from their anticancer activity[54]; terpenes are also known anticancer, antioxidant, and anti-inflammatory agents[55], flavonoids also possess antibacterial and antioxidant activities in addition to their antifungal and antiviral effects[56], and tannins were reported to possess antiviral, insecticidal, and antibacterial activity to name a few[57]. Among these bioactive phytochemicals widely known to have pesticide activities are flavonoids, alkaloids, and terpenes (sesquiterpenes, diterpenes, and monoterpenes) [58-60]. Flavonoids exhibit a broad spectrum of pesticidal activity as it targets a wide range of organisms, from fungi and bacteria to insects, algae, mollusks, and plants [60]. On another note, alkaloids like oxymatrine from Sophora flavescens and 10-O-dmethyl-17-O-methylisoarnottianamide and 6-acetonyl-N-methyl-dihydrodecarine from Zanthoxylum lemairei, exhibits target-specific insecticidal activity61 and larvicidal activity against malaria-vector Anopheles gambiae mosquito [61], respectively. Furthermore, terpenes found in Tithonia diversifolia plants exhibit biological activity against insect pests and antifeedant activity against
arthropod pests [61]. This study's results showed that pesticide phytochemicals alkaloids and flavonoids were also present in the three plant parts of Pittosporum molucannum. However, further investigation should be made to confirm that these compounds are the main contributors to the pesticidal activity of the plant. In addition, although various authors report the presence of flavonoids, phenols, alkaloids, lignins, anthraquinones, steroids, tannins, saponins, fixed oils, and glycosides in different species of Pittosporum plant [62], this study is the first to report that P. molucannum plant contains alkaloids, flavonoids, phenolics, and tannins.
Toxicity Profile of the different P. moluccanum extracts
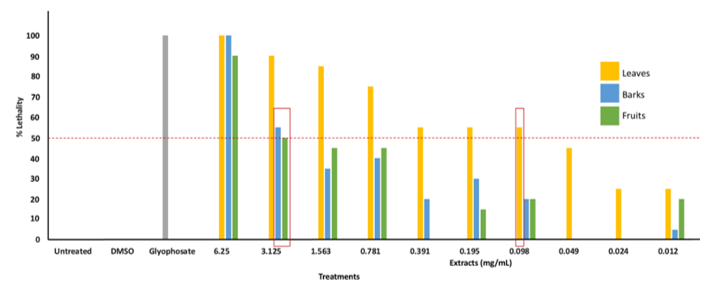
The toxicity of the plant extracts was determined through brine shrimp lethality assay (LC50) and MTT cytotoxicity assay (CC50) using Saccharomyces cerevisiae as the test organism. Brine shrimp lethality assay was done to determine the concentration of the extracts that could kill more than 50 % of the napauli population, and MTT assay was done to determine the concentration that could inhibit more than 50 % of the growth of yeast cells. Results of the lethality assay showed that the leaves extract was lethal (LC50) to the napauli at 0.098 mg/mL concentration exhibiting 55 % lethality (± 2.5 SD). The barks and fruits were found lethal to the napauli at 3.125 mg/mL exhibiting 55 % (± 1.5 SD) and 50 % (± 1.0 SD), respectively. Moreover, positive control glyphosphate (3 mg/mL) exhibits 100 % (± 0.00 SD) lethality against the test organisms. (Fig. 2) These results were classified according to Clarkson's toxicity criterion to describe their respective toxicity. Extracts with LC50 of greater than 1000 μg/ml are considered nontoxic, extracts with LC50 of 500 - 1000 µg/ml are low toxic, extracts with LC50 of 100 - 500 μg/ml are medium toxic, while extracts with LC50 of 0 - 100 µg/ml are highly toxic [63]. According to the criterion, P. molucanum leaves were highly toxic, while the fruits and barks were nontoxic.
Results of MTT assay suggest that the leaves exhibit 70.15 % cytotoxicity ± 0.22 SD (CC50) at 0.2 ug/mL, the barks exhibit 64.98 % ± 0.38 SD (CC50) at the same concentration of 0.2 ug/mL, while the fruits exhibit 69.28 % ± 0.12 SD (CC50) at 0.39 ug/mL (Figure 3).
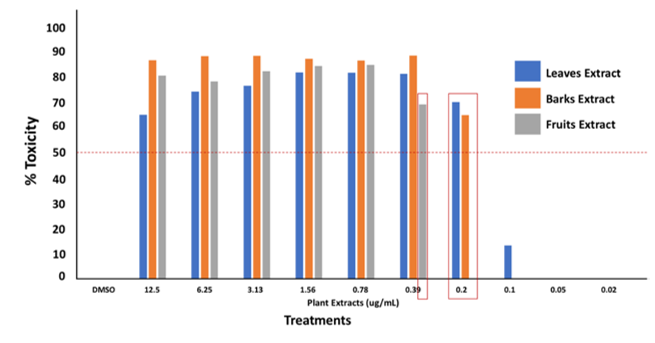
Pittosporum molucannum is one of the rarely explored species among the Pittosporum genus. It was reported to have antimalarial activity18, and antioxidant activity by scavenging DPPH, OH, and superoxide radicals at 14.9 µg/mL, 1.24 µg, and 223.3 µg/mL, respectively [32]. Despite these findings, its toxicity was not yet reported. Rather, only toxicity of related species was established. To give a few examples, the different solvent extracts of P. phylliraeoides and P. angustifolium extract (at 1 mg/mL concentration) were reported to be nontoxic (less than 50 % lethality) against Artemia franciscana napauli [64,65]. More so, P. angustifolium extracts are also nontoxic at 200 µg/mL concentration evaluated using MTS (3-(4,5-dimethylthiazol-2-yl)-5- (3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)) [66]. In addition, compounds extracted from P. tobira were also found to not affect the cell viability of NIH/3T3 cells at different concentrations (50-400 µg/mL) as determined using MTT assay, thus reported to be nontoxic [67]. On the other note, these reports were found to be contrary to the findings of this study. Ethanolic extracts of P. molucannum leaves, fruits, and bark are considered toxic to the test organism. At lower concentrations of 0.2 and 0.39 µg/mL, the extracts were already considered cytotoxic.
Molluscicidal activity determination of the different plant extracts
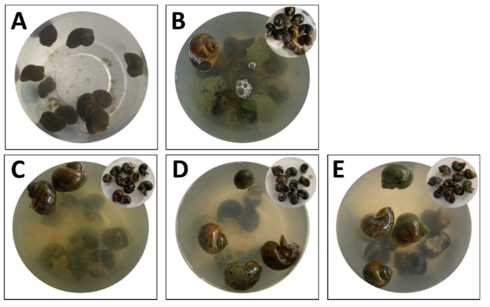
This test was done to determine the molluscicide effect of P. molucannum plant extracts on golden apple snails (P. canaliculata). Briefly, increasing concentrations of the plant extracts (10x CC50, 15x CC50, 50x CC50, 250 µg/mL, and 500 µg/mL) were used in the experiment and compared to the activity of metaldehyde (6 µg/mL) as the positive control and DMSO as the negative control. Ten snails per group were assigned to each treatment. After 24 h exposure, dead snails were identified, and percent mortality was calculated. Dead snails were observed to be (a) not moving, (b) have shriveled feet that extrude out of the shell, or (c) their mantle collapsed so that it could be easily evicted from their shells [66,68].
The results of this experiment showed that the plant extracts were able to kill golden apple snails, similar to metaldehyde (6 µg/mL). Snails treated with the plant extracts were mostly not moving and had shriveled feet that extruded out of the shell, as snails treated with metaldehyde have mostly collapsed mantles facilitating the easy eviction of the snails out of their shells (Figure 4).
Moreover, results suggest that the plant extracts exhibit molluscicide activity in a concentration-dependent manner (Fig. 5). No mortality against golden apple snails was observed on DMSO (negative control) and the plant extracts at 10x CC50 concentrations as compared to the 71.11 % (± 1.33 SD) mortality of 6 µg/mL metaldehyde (positive control). At 15x CC50 and 250 µg/mL
concentrations, minimal activity (below the 50% threshold) was observed on leaves and bark extracts only. Notedly, the fruit extract exhibits minimal activity at 15x CC50 concentration but increases significantly to 96.47 % (± 0.47 SD) mortality at 250 µg/mL concentration. At 500 µg/mL concentration, the three extracts exhibited 100 % mortality against golden apple snails.
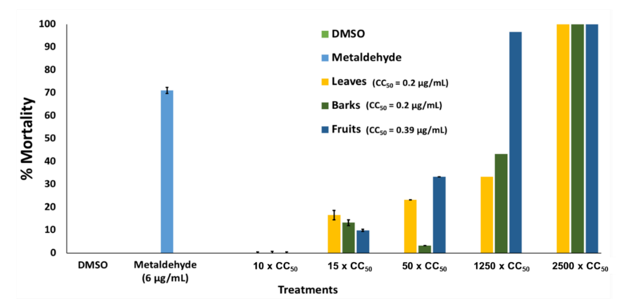
The application of molluscicides is an efficient measure to control snail infestation in agricultural lands such as rice and wheat field. Several synthetic compounds have already been developed for the control of these pests, such as metaldehyde, niclosamide, and methiocarb, to name a few; however, large-scale use imposes toxicity on other non-target organisms and the environment [69]. Because of the harmful effects of synthetic molluscicidal compounds, this study prompted to search for efficient natural biopesticides that could control agricultural pest infestation that is nontoxic to non-target organisms and the environment. The genus Pittosporum has long been claimed to have pesticidal activity, particularly against snails (molluscicidal activity). In fact, several species were reported to exhibit molluscicidal activity. Examples of these species were P. tobira plant dry powder with an LC90 at 120 ppm against Biomphalaria alexandrina snails [24], and P. undulatum essential oil that exhibits 10 % (flowers and leaves) mortality against adult Radix peregra at 100 ppm. Moreover, P. undulatum exhibits an ovicidal effect (4.2% of hatching), calculated as the percentage of egg hatching at 100 ppm concentration [70]. This reported activity was found congruent to the findings of this study. Pittosporum molucannum which is a related species of P. tobira
and P. undulatum was found to cause lethality at 1250x and 2500x its CC50 values against P. canaliculata. This further suggests the potential of P. molucannum plant as a molluscicide and as a source of natural biopesticides.
Herbicidal activity of the different P. molucannum extracts
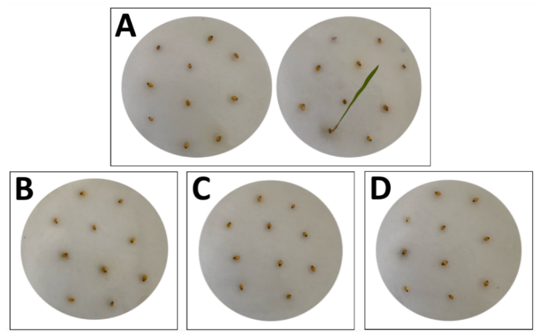
This experiment was performed following the methods of Feng and coworkers with a few modifications, such as natural photoperiod of night and day was utilized and seed germination was counted after 7 days of cultivation [52]. Here, ten seeds were grouped and exposed to 1 µg/mL glyphosate (positive control), leaves extract (2 µg/mL), fruit extract (3.9 µg/mL), and bark extract (2 µg/mL) accordingly. After 7 days, shoot growth and root length were identified and recorded. Percent (%) inhibition of seed germination was then calculated, and the length of the grown shoot and root per seed was recorded.
The result of the experiment suggests that the plant extracts (leaves, fruits, and barks) at 10x CC50 concentration inhibited seed germination of the barnyard weeds since no root or shoot length was observed on the treated seeds (Figure 6).
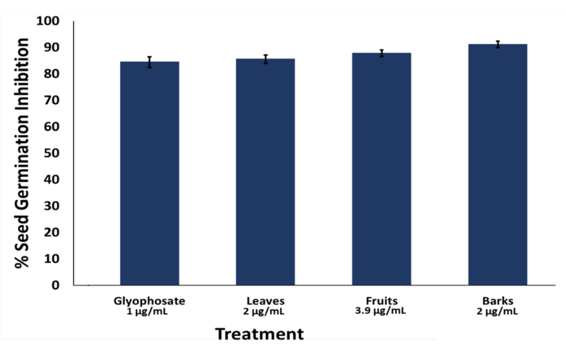
Moreover, % inhibition of seed germination suggests that the extract exhibit similar activity with glyphosate (Fig. 7). For the positive control (1 µg/mL glyphosate), 84.44 % ± 1.57 SD inhibition of seed germination was observed while 85.56 % (± 1.20 SD), 87.78 % (± 1.22 SD), and 91.11 % (± 1.28 SD) was observed on the leaves, fruits, and bark extracts, respectively.
Just like other pesticides, the continued use of synthetic herbicides in agricultural and non-agricultural plants poses health risks and pollution [71]. Because of this problem, several plants have been investigated for their allelopathic characteristics that either stimulate or inhibit the growth of their neighboring plant species as these allelochemicals play an important role in the ecological function of the plant [72,73]. Among these allelochemicals, terpenoids, phenolics, and nitrogen-containing compounds such as alkaloids were reported as the plant defense systems against external factors and plant growth inhibitors [73,74]. Examples of plants producing allelochemicals are the Pachyptera hymenaea, Anomianthus dulcis, and Ficus macrocarpa, Agapetes lobbii among others. These plants were observed to have strong (more than 50 %) inhibitory effects on germination and early seedling growth of radish and barnyardgrass due to their higher phenolic and flavonoid contents [73]. Among the Pittosporum plants, P. tobira fruits and leaves were reported to have herbicidal activity by exhibiting more than 60 % inhibition of root growth of barnyardgrass seedlings at 1000 µg/mL. The result of this experiment was found congruent with the report of Kim and Dong [74]. P. molucannum exhibits inhibition of the root growth of barnyardgrass in a smaller concentration than P. tobira. The ethanolic extract of P. molucannum leaves, barks, and fruits exhibit 85-91 % root growth inhibition at 2 µg/mL (leaves and barks) and 3.9 µg/mL (fruits) accordingly. Moreover, this result further supports the plant's potential as a source of natural biopesticides.
Conclusion
The preliminary findings reported in this study support the potential of Pittosporum molucannum plant extracts as a source of natural biopesticides that could be used for the control of pests such as golden apple snails and barnyardgrass.
References
- Boedeker W, Watts M, Clausing P, et al. (2020). The global distribution of acute unintentional pesticide poisoning: estimations based on a systematic review. BMC Public Health. 20(1):1875.
View at Publisher | View at Google Scholar - Mew EJ, Padmanathan P, Konradsen F, et al. (2017). The global burden of fatal self-poisoning with pesticides 2006-15: Systematic review. J Affect Disord. 219: 93-104.
View at Publisher | View at Google Scholar - Lu JL, Cosca KZ, Del Mundo J. (2010). Trends of pesticide exposure and related cases in the Philippines. J Rural Med. 5(2): 153-164.
View at Publisher | View at Google Scholar - Gilden RC, Huffling K, Sattler B. (2010). Pesticides and health risks. J Obstet Gynecol Neonatal Nurs. 39(1): 103-110.
View at Publisher | View at Google Scholar - Lee GH, Choi KC. (2020). Adverse effects of pesticides on the functions of immune system. Comp Biochem Physiol C Toxicol Pharmacol. 235: 108789.
View at Publisher | View at Google Scholar - Kim KH, Kabir E, Jahan SA. (2017). Exposure to pesticides and the associated human health effects. Sci Total Environ. 575: 525-535.
View at Publisher | View at Google Scholar - Casida JE, Durkin KA. (2017). Pesticide Chemical Research in Toxicology: Lessons from Nature. Chem Res Toxicol. 30(1): 94-104.
View at Publisher | View at Google Scholar - Corsini E, Sokooti M, Galli CL, et al. (2013). Pesticide induced immunotoxicity in humans: a comprehensive review of the existing evidence. Toxicology. 307: 123-135.
View at Publisher | View at Google Scholar - Wickerham EL, Lozoff B, Shao J, et al. (2012). Reduced birth weight in relation to pesticide mixtures detected in cord blood of full-term infants. Environ Int. 47: 80-85.
View at Publisher | View at Google Scholar - Van Maele-Fabry G, Lantin AC, Hoet P, et al. (2011). Residential exposure to pesticides and childhood leukaemia: a systematic review and meta-analysis. Environ Int. 37(1): 280-291.
View at Publisher | View at Google Scholar - Yang EC, Chuang YC, Chen YL, et al. (2008). Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J Econ Entomol. 101(6): 1743-1748.
View at Publisher | View at Google Scholar - Werner I, Schneeweiss A, Segner H, et al. (2021). Environmental Risk of Pesticides for Fish in Small- and Medium-Sized Streams of Switzerland. Toxics. 9(4).
View at Publisher | View at Google Scholar - Mahmood I, Imadi SR, Shazadi K, et al., editors. (2016). Effects of Pesticides on Environment.
View at Publisher | View at Google Scholar - Council UN-HR. (2017). Report of the Special Rapporteur on the right to food: United Nations.
View at Publisher | View at Google Scholar - Jafarbeigi F, Samih MA, Zarabi M, et al. (2012). The effect of some herbal extracts and pesticides on the biological parameters of Bemisia tabaci (Genn.) (Hem.: Aleyrodidae) pertaining to tomato grown under controlled conditions. Journal of Plant Protection Research. 52: 375-380.
View at Publisher | View at Google Scholar - Nau GWaB, Maria Novita Inya. (2020). AJCB-Vol9-No2-Nau-Buku.pdf. Asian journal of Conservation Biology. 9(2): 214-220.
View at Publisher | View at Google Scholar - Batuyong MAR, Calaramo MA, Alejandro GJD. (2020). A checklist and conservation status of vascular plants in the Limestone Forest of Metropolitan Ilocos Norte Watershed Forest Reserve, Northwestern Luzon, Philippines. Biodiversitas. 21.
View at Publisher | View at Google Scholar - Cayzer LW, Chandler GT. (2018). Pittosporum ridleyi (Pittosporaceae), a new name for the ‘rusty-leaved’ Pittosporum in Malaysia. Gardens' Bulletin Singapore.
View at Publisher | View at Google Scholar - Nyongbela KD, Lannang AM, Ayimele GA, et al. (2013). Isolation and identification of an antiparasitic triterpenoid estersaponin from the stem bark of Pittosporum mannii (Pittosporaceae). Asian Pacific Journal of Tropical Disease. 3(5): 389-392.
View at Publisher | View at Google Scholar - Madikizela B, McGaw LJ. (2017). Pittosporum viridiflorum Sims (Pittosporaceae): A review on a useful medicinal plant native to South Africa and tropical Africa. J Ethnopharmacol. 205: 217-230.
View at Publisher | View at Google Scholar - Okoye TC, Uzor PF, Onyeto CA, et al., editors. (2014). Safe African Medicinal Plants for Clinical Studies.
View at Publisher | View at Google Scholar - Seo Y, Berger JM, Hoch J, et al. (2002). A new triterpene saponin from Pittosporum viridiflorum from the Madagascar rainforest. J Nat Prod. 65(1):65-68.
View at Publisher | View at Google Scholar - Ibrahim AM, Abdel-Gawad MM, El-Nahas HA, et al. (2015). Studies on the molluscicidal activity of Agave angustifolia and Pittosporum tobira on schistosomiasis transmitting snails. Journal of the Egyptian Society of Parasitology. 45, 1: 133-41.
View at Publisher | View at Google Scholar - Bäcker C, Drwal MN, Preissner R, et al. (2016). Inhibition of DNA-Topoisomerase I by Acylated Triterpene Saponins from Pittosporum angustifolium Lodd. Nat Prod Bioprospect. 6(2): 141-147.
View at Publisher | View at Google Scholar - Bäcker C, Jenett-Siems K, Siems K, et al. (2014). Cytotoxic Saponins from the Seeds of Pittosporum angustifolium. Zeitschrift für Naturforschung C. 69: 191 - 198.
View at Publisher | View at Google Scholar - Sadgrove NJ, Jones GL. (2013). Chemical and biological characterisation of solvent extracts and essential oils from leaves and fruit of two Australian species of Pittosporum (Pittosporaceae) used in aboriginal medicinal practice. J Ethnopharmacol. 145(3):813-21.
View at Publisher | View at Google Scholar - Shyamal S, Latha PG, Shine VJ, et al. (2006). Hepatoprotective effects of Pittosporum neelgherrense Wight&Arn., a popular Indian ethnomedicine. J Ethnopharmacol. 107(1): 151-155.
View at Publisher | View at Google Scholar - Gunsai KK, Gandhi AJ, Acharya R, et al. (2020). Ethnomedicinal Uses, Phytochemistry and Pharmacological Activities of Pittosporum floribundum Wight. & Arn. – A Review. European journal of medicinal plants. 48-55.
View at Publisher | View at Google Scholar - Muhamood M, Maya S, G P, et al. (2014). Medicinal Properties of Pittosporum and It's Applicability in Oral Lesions. Universal Journal of Pharmacy. 12: 3-45.
View at Publisher | View at Google Scholar - Lee MH, Jiang CB, Juan SH, et al. (2006). Antioxidant and heme oxygenase-1 (HO-1)-induced effects of selected Taiwanese plants. Fitoterapia. 77(2): 109-115.
View at Publisher | View at Google Scholar - Rohman F, Juma Y, Sulisetijono S, et al. (2019). Plants diversity as a medicinal plant by the Tengger Tribe, Bromo Tengger Semeru National Park, East Java, Indonesia. Eurasian Journal of Medicine. 13:2293-2298.
View at Publisher | View at Google Scholar - Taek M, Prajogo B, Agil M. (2018). Plants used in traditional medicine for treatment of malaria by Tetun ethnic people in West Timor Indonesia. Asian Pacific Journal of Tropical Medicine. 11: 630-637.
View at Publisher | View at Google Scholar - Setiyarini CT, Kristiani EBE, Yulianto S, editors. (2020). The Study of Morphology, Phytochemical, and Distribution of Pittosporum moluccanum in Mount Merbabu National Park (TNGM)For the Development of Educationl Teaching Materials.
View at Publisher | View at Google Scholar - Farahmandfar R, Esmaeilzadeh Kenari R, Asnaashari M, et al. (2019). Bioactive compounds, antioxidant and antimicrobial activities. Food Sci Nutr. 7(2): 465-475.
View at Publisher | View at Google Scholar - Lee H-W, Lee H-S. (2015). Acaricidal potency of active constituent isolated from Mentha piperita and its structural analogs against pyroglyphid mites. Journal of the Korean Society for Applied Biological Chemistry. 58: 597-602.
View at Publisher | View at Google Scholar - Nunes JC, Lago MG, Castelo-Branco VN, et al. (2016). Effect of drying method on volatile compounds, phenolic profile and antioxidant capacity of guava powders. Food Chem. 197(Pt A): 881-90.
View at Publisher | View at Google Scholar - Nugroho AE, Akbar FF, Wiyani A, et al. (2015). Cytotoxic Effect and Constituent Profile of Alkaloid Fractions from Ethanolic Extract of Ficus septica Burm. f. Leaves on T47D Breast Cancer Cells. Asian Pac J Cancer Prev. 16(16): 7337-7342.
View at Publisher | View at Google Scholar - Sinhababu A, Basu S, Dey H. (2015). Modified ninhydrin reagents to detect amino acids on TLC plates. Research on Chemical Intermediates. 41(5): 2785-2792.
View at Publisher | View at Google Scholar - La H, Aktsar Roskiana A, Ahmad N. (2018). Qualitative and Quantitative Test of Total Flavonoid Buni Fruit (Antidesma bunius (L.) Spreng) with UV-Vis Spectrophotometry Method. Pharmacognosy Journal. 10(1).
View at Publisher | View at Google Scholar - Ervina M, Nawu YE, Esar SY. (2016). Comparison of in vitro antioxidant activity of infusion, extract and fractions of Indonesian Cinnamon (Cinnamomum burmannii) bark. 23: 1346-1350.
View at Publisher | View at Google Scholar - Gerlach A, Gadea A, Silveira R, et al. (2018). The Use of Anisaldehyde Sulfuric Acid as an Alternative Spray Reagent in TLC Analysis Reveals Three Classes of Compounds in the Genus Usnea Adans. (Parmeliaceae, lichenized Ascomycota).
View at Publisher | View at Google Scholar - Santana ÁL, Johner JCF, Meireles MAA, editors. (2016). Thin-Layer Chromatography Profile of Annatto Extracts Obtained with Supercritical Carbon Dioxide and Subsequently High-Pressure Phase Equilibrium Data.
View at Publisher | View at Google Scholar - Das K, Gezici S. (2018). Secondary plant metabolites, their separation and identification, and role in human disease prevention. Annals of Phytomedicine: An International Journal. 7: 13-24.
View at Publisher | View at Google Scholar - Asghari-Paskiabi F, Imani M, Rafii-Tabar H, et al. (2019). Physicochemical properties, antifungal activity and cytotoxicity of selenium sulfide nanoparticles green synthesized by Saccharomyces cerevisiae. Biochem Biophys Res Commun. 516(4):1078-1084.
View at Publisher | View at Google Scholar - Choudhary DK, Mishra A. (2018). In vitro investigation of hypoglycemic and oxidative stress properties of fava bean (Vicia faba L.) seed extract in Saccharomyces cerevisiae 2376. Prep Biochem Biotechnol. 48(10): 920-929.
View at Publisher | View at Google Scholar - Galván Márquez I, Ghiyasvand M, Massarsky A, et al. (2018). Zinc oxide and silver nanoparticles toxicity in the baker's yeast, Saccharomyces cerevisiae. PLoS One. 13(3): e0193111.
View at Publisher | View at Google Scholar - Waghulde S, Kale MK, Patil V. (2019). Brine Shrimp Lethality Assay of the Aqueous and Ethanolic Extracts of the Selected Species of Medicinal Plants. Proceedings. 41(1):47.
View at Publisher | View at Google Scholar - Organization) WWH. (2019). Guidelines for laboratory and field testing of molluscicides for control of schistosomiasis. Geneva: World Health Organization.
View at Publisher | View at Google Scholar - Gomez DC, Anacta N. (2020). A New Method to Test Molluscicides against the Philippine Schistosomiasis Snail Vectors. J Parasitol Res. 3827125.
View at Publisher | View at Google Scholar - Liao M, Xiao JJ, Zhou LJ, et al. (2016). Insecticidal Activity of Melaleuca alternifolia Essential Oil and RNA-Seq Analysis of Sitophilus zeamais Transcriptome in Response to Oil Fumigation. PLoS One. 11(12): e0167748.
View at Publisher | View at Google Scholar - Shao H, Huang X, Wei X, et al. (2012). Phytotoxic effects and a phytotoxin from the invasive plant Xanthium it alicum Moretti. Molecules. 17(4): 4037-4046.
View at Publisher | View at Google Scholar - Feng G, Chen M, Ye H-C, et al. (2019). Herbicidal activities of compounds isolated from the medicinal plant Piper sacramentum. Industrial Crops and Products. 132: 41-47.
View at Publisher | View at Google Scholar - Lin D, Xiao M, Zhao J, et al. (2016). An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules. 21(10).
View at Publisher | View at Google Scholar - Ashihara H. (2015). Occurrence, Biosynthesis and Metabolism of Theanine (γ-Glutamyl-L-Ethylamide) in Plants: A Comprehensive Review. Natural Product Communications. 10(5):1934578X1501000525.
View at Publisher | View at Google Scholar - Paduch R, Kandefer-Szerszeń M, Trytek M, et al. (2017). Terpenes: substances useful in human healthcare. Arch Immunol Ther Exp (Warsz). 55(5):315-27.
View at Publisher | View at Google Scholar - Panche AN, Diwan AD, Chandra SR. (2016). Flavonoids: an overview. J Nutr Sci. 5: e47.
View at Publisher | View at Google Scholar - Girard M, Bee G. (2020). Invited review: Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. Animal. 14(1): 95-107.
View at Publisher | View at Google Scholar - Schnarr L, Segatto ML, Olsson O, et al. (2022). Flavonoids as biopesticides - Systematic assessment of sources, structures, activities and environmental fate. Sci Total Environ. 824:153781.
View at Publisher | View at Google Scholar - Kerebba N, Oyedeji AO, Byamukama R, et al. (2019). Pesticidal activity of Tithonia diversifolia (Hemsl.) A. Gray and Tephrosia vogelii (Hook f.); phytochemical isolation and characterization: A review. South African Journal of Botany. 121:366-376.
View at Publisher | View at Google Scholar - Damalas CA, Koutroubas SD. (2018). Current Status and Recent Developments in Biopesticide Use. Agriculture. 8(1):13.
View at Publisher | View at Google Scholar - Talontsi FM, Matasyoh JC, Ngoumfo RM, et al. (2011). Mosquito larvicidal activity of alkaloids from Zanthoxylum lemairei against the malaria vector Anopheles gambiae. Pesticide Biochemistry and Physiology. 99(1):82-85.
View at Publisher | View at Google Scholar - Chakkinga Thodi R, Pillai A, M K, et al. (2019). A review on phytochemical, ethnomedicinal and pharmacological studies of genus Pittosporum (Pittosporaceae), in India. Journal of Pharmacognosy and Phytochemistry. 155-162.
View at Publisher | View at Google Scholar - Anaya-Esparza LM, González-Silva N, Yahia EM, et al. (2019). Effect of Tio. Nanomaterials (Basel). 9(7).
View at Publisher | View at Google Scholar - Communications P, Vesoul J, Cock I. (2011). An Examination of the Medicinal Potential of Pittosporum Phylliraeoides: Toxicity, Antibacterial and Antifungal Activities INTRODUCTION. Pharmacognosy Communications. 1:8-17.
View at Publisher | View at Google Scholar - Blonk B, Cock IE. (2019). Interactive antimicrobial and toxicity profiles of Pittosporum angustifolium Lodd. extracts with conventional antimicrobials. J Integr Med. 17(4): 261-272.
View at Publisher | View at Google Scholar - Feitosa dos Santos A, Ferraz PcAL, Ventura Pinto A, et al. (2000). Molluscicidal activity of 2-hydroxy-3-alkyl-1,4-naphthoquinones and derivatives. International Journal for Parasitology. 30(11): 1199-1202.
View at Publisher | View at Google Scholar - Oh JH, Jeong YJ, Koo HJ, et al. (2014). antimicrobial activities against periodontopathic bacteria of Pittosporum tobira and its active compound. Molecules. 19(3): 3607-3616.
View at Publisher | View at Google Scholar - Santos CCS, Araújo SS, Santos ALLM, et al. (2014). Evaluation of the toxicity and molluscicidal and larvicidal activities of Schinopsis brasiliensis stem bark extract and its fractions. Revista Brasileira de Farmacognosia. 24(3): 298-303.
View at Publisher | View at Google Scholar - He P, Wang W, Sanogo B, et al. (2017). Molluscicidal activity and mechanism of toxicity of a novel salicylanilide ester derivative against Biomphalaria species. Parasit Vectors. 10(1): 383.
View at Publisher | View at Google Scholar - Teixeira T, Rosa JS, Rainha N, et al. (2012). Assessment of molluscicidal activity of essential oils from five Azorean plants against Radix peregra (Müller, 1774). Chemosphere. 87(1): 1-6.
View at Publisher | View at Google Scholar - Poonpaiboonpipat T, Jumpathong J. (2019). Evaluating herbicidal potential of aqueous-ethanol extracts of local plant species against Echinochloa crus-galli and Raphanus sativus. International Journal of Agriculture and Biology. 21: 648-652.
View at Publisher | View at Google Scholar - Jilani G, Mahmood S, Chaudhry AN, et al. (2008). Allelochemicals: sources, toxicity and microbial transformation in soil —a review. Annals of Microbiology. 58(3): 351-357.
View at Publisher | View at Google Scholar - Fukuta M, Dang Xuan T, Deba F, et al. (2007). Comparative efficacies in vitro of antibacterial, fungicidal, antioxidant, and herbicidal activities of momilatones A and B. Journal of Plant Interactions. 2(4): 245-251.
View at Publisher | View at Google Scholar - Kim K-W, Lee D-G. (2007). Screening of Herbicidal and Fungicidal Activities from Resource Plants in Korea. Korean Journal of Weed Science.
View at Publisher | View at Google Scholar

 Clinic
Clinic
