Review Article | DOI: https://doi.org/10.31579/2834-8532/018
Immunogenetics of Stem Cells: A Challenge
Ex-Head, Department of Medical Genetics, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow 226014 (UP), India.
*Corresponding Author: Suraksha Agrawal, Ex-Head, Department of Medical Genetics, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow 226014 (UP), India.
Citation: Suraksha Agrawal, (2023), Immunogenetics of Stem Cells: A Challange, Clinical Genetic Research, 2(2); Doi:10.31579/2834-8532/018
Copyright: © 2023 Suraksha Agrawal, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 11 April 2023 | Accepted: 21 April 2023 | Published: 14 March 2023
Keywords: stem cells; micro-RNA; human leukocyte antigens; stem cell therapy
Abstract
A population of undifferentiated cells known as stem cells have a high proliferative capacity (self-renewal), typically developing from a single cell (clonal), differentiating into several cell and tissue types (potent). There are several sources of stem cells with varied potencies. Pluripotent cells are developed from the inner cell mass of the embryo by embryonic stem cells, and induced pluripotent cells are created by reprogramming somatic cells. Endoderm, mesoderm, and ectoderm are the three germ layers from which pluripotent cells can develop into tissue. In cellular treatment, stem cells can be employed to rebuild organs or replace damaged cells. Furthermore, stem cells have improved our knowledge of both disease causation and development. Moreover, disease-specific cell lines can be developed and employed in research. Many of these restrictions are being overcome, though, and this could result in significant advancements in the treatment of disease. In this review, the topic of stem cells is introduced, along with the definition, history, and classification of these cells, as well as their use in regenerative medicine. The immunogenetic aspect of stem cells has also been focused on extensively in this review.
Summary
The fertilized egg divides into two daughter cells that further undergo cell division and the resultant cells differentiate into daughter cells. Stem cells are different from other cells of the body because stem cells can self-renew: make copies of themself and differentiate into mother types of cells which are highly specialized cells of the body and are the master cells. These cells can be differentiated into blood cells, nerve cells, and muscle cells. Interestingly the specialized cells cannot divide to make copies of themselves. This makes stem cells very important. The body needs stem cells to replace specialized cells that die or are damaged or get used up. Self-renewal is necessary because if the stem cells didn’t copy themselves, they will quickly run out. It is vital for the body to maintain a pool of stem cells to use throughout life. The human body needs 100,000 million new blood cells every day. Of course, differentiation is also important for making all different kinds of cells in the body during the development of an embryo from a single fertilized egg. Specialized cells like skin, red blood or gut cells cannot undergo mitosis, hence there is a need for stem cells. There are a few exceptions (e.g., liver cells or T-cells) but generally dedicated cells can no longer undergo cell division. The progenitor’s cells can divide to allow a large number of new cells to be made. In the human body, cells don’t generally divide to produce one stem cell and one specialized cell at the same time. In fruit flies, stem cells can divide to make one stem cell and a specialized cell.
There are different types of stem cells e.g. embryonic stem cells are found in the blastocyst, a very early-stage embryo that has about 50 to 100 cells; Tissue stem cells are found in the tissues of the body (in a fetus, baby, child or adult). These cells are taken from the inside of the blastocyst, a very early stage of the embryo (Figure 1).
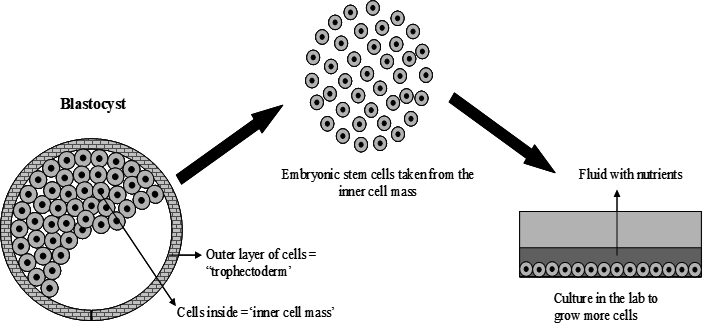
Figure 1: Stem cell niche. Microenvironment around stem cell that trigger the cell division and differentiation into different lineages
The blastocyst is a ball of about 50-100 cells, however, not implanted in the womb. It is composed of an outer layer of cells, a fluid-filled space and a group of cells called the inner cell mass. ES cells are found in the inner cell mass ES contains so much information that it can manifest approximately 100 trillion cells that make up the adult human organism. Stem cells remain quiescent (non-dividing) for many years until they are activated once activated they can develop into different organs. Some types of adult stem cells have the ability to differentiate into a number of different cell types if given the appropriate environment [1]. Embryonic stem cells can make different types of cells in the body these are termed pluripotent cells (Figure 2).
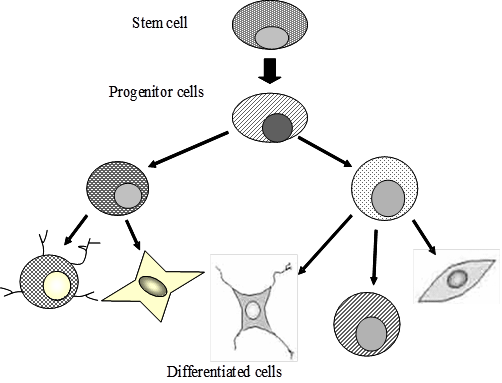
Figure 2: Pluripotency of stem cells: Stem cells are able to give rise to a variety of cell lineages, each of which is committed progenitor for a differentiated cell type
Stem cells are found in very small populations in the human body i.e., ~1 stem cell in 100,000 cells in circulating blood.
After several decades of experiments, stem cell therapy is becoming a magnificent game changer for medicine [2]. In the 1960s, researchers discovered that the bone marrow contains at least two types of stem cells. The hematopoietic stem cells form all types of blood cells in the body. A second population, called bone marrow stromal cells were discovered a few years later stromal cells are a mixed cell population that generates bone, cartilage, fat, and fibrous connective tissues. Since its discovery, the most widely studied example of adult stem cells is hematopoietic stem cells (HSCs), which sustain the formation of the blood and immune system throughout life.
The bone marrow compartment is largely made up of dedicated progenitor cells, non-circulating stromal cells that have the ability to develop into mesenchymal lineages (termed mesenchymal stem cells), and HSCs. Stem cell surface markers The stem cells are characterized on the basis of stem cell markers. These markers are specialized proteins called receptors that have the capability of selective binding or adhering to other "signalling" molecules. The cell surface that has gathered immense interest is sialomucin CD34, which was initially found to be expressed in a small fraction of human bone marrow cells The CD34+ cells of marrow or mobilized peripheral blood are responsible for most of the hematopoietic activity CD34 has therefore been considered to be the most critical marker for hematopoietic stem cells (HSCs). CD34 expression on primitive cells is downregulated as they differentiate into mature cells [3]. It has been reported that during the differentiation of MUTZ-3 cells into Langerhans cells, expression of RTN1A mRNA and protein preceded established Langerhans cell markers CD1a and CD207, and RTN1A protein partially co-localized with the endoplasmic reticulum marker protein disulfide isomerase. The RTN1A is found to be expressed in approximately 80% of Langerhans cell precursors in human embryonic skin. The tRTN1A is a marker for cells of the dendritic lineage, including Langerhans cells and dermal dendritic cells. This finding elucidation the elusive functional roles of RTN1A in both the immune and the nervous systems [4]. Recent work on the cell lines has proved that CD34 human progenitor cell line Mutz-3 can be differentiated into functional Osteoclasts. Another surface marker is CD133, which is a 120-kDa glycosylated protein with five transmembrane domains. Recent literature has shown that CD133 expression is not restricted to primitive blood cells, but is also present in unique cell populations of non-hematopoietic origin and hence considered to be a mature marker [5] CD133+ progenitor cells from peripheral blood can be induced to differentiate into endothelial cells in vitro.
ABCG2 was identified in the breast cancer cell line it is an ATP-binding cassette superfamily G member 2. It is a determinant of the wide variety of stem cells, including HSC. It is located in the plasma membrane. The ABCG2 expression can undergo alterations under different pathophysiological conditions such as inflammation, infection, tissue injury, disease pathologyand in response to xenobiotics and endobiotic.
These alterations may restrict the bioavailability of therapeutic substrate drugs that may cause drug resistance and in certain cases deteriorate the pathophysiological state and aggravate its severity. Considering the vital role of ABCG2 in normal physiology, therapeutic interventions directly targeting the transporter function may produce severe side effects. This advocates the modulation of transporter regulation instead of inhibiting the transporter itself. This will allow subtle changes in ABCG2 activity. His requires a thorough comprehension of diverse factors and complex signalling pathways (Kinases, Wnt/β-catenin, Sonic Hedgehog) operating at multiple regulatory levels dictating ABCG2 expression and activity [6].
Sca-1 (stem cell antigen 1, Ly-6A/E),
Sca-1 (stem cell antigen 1, Ly-6A/E), is an 18-kDa phosphatidylinositol-anchored protein member of the Ly-6 antigen family. The Sca-1 is the most recognized HSC marker in mice with both Ly-6 haplotypes as it is expressed on multipotent HSCs. Sca-1 has also been discovered in several non-hematopoietic tissues also, however, can be used to enrich progenitor cell populations other than HSCs [7]. Most stem cell markers are given short names based on the molecules that bind to the stem cell surface receptors. One such example is the cell that has the receptor stem cell antigen -1, on its surface, which is identified as Sca-1. A combination of multiple markers is used to identify a particular stem cell type. For example, a special type of hematopoietic stem cell from blood and bone marrow called "side population" or "SP" is described as (CD34-/low, c-Kit+, Sca-1+).
The markers on the cells are identified by using fluorescent tags using flow cytometry. A second method is based on stem cell markers and their fluorescent tags to visually assess cells as they exist in tissues by using a microscope.
How cells become specialized in the organism's development has been solved by identifying genes and transcription factors which may be unique to stem cells. For example, a gene marker called PDX-1 is specific for a transcription factor protein that initiates activation of the insulin gene and is capable of the development of islet cells in the pancreas [8]. It has been reported that adult stem cells are lineage-restricted, but recent observations demonstrate that bone marrow-derived myogenic progenitors participate in regenerating damaged skeletal muscle and ischemic myocardium. Muscle stem cells are shown to contribute to haematopoiesis although this could relate to their common embryological origin because both blood and muscle cells are derived from the mesoderm. The details of markers used for stem cell differentiation are shown in Table 1.
| Marker name | Cell type |
| Blood vessel | |
| Fetal liver kinase-1 (Flk1) | Endothelial |
| Smooth muscle cell-specific myosin heavy chain | Smooth muscle |
| Vascular endothelial cell cadherin | Smooth muscle |
| Bone | |
| Bone-specific alkaline phosphatase (BAP) | Osteoblast |
| Hydroxyapatite | Osteoblast |
| Osteocalcin (OC) | Osteoblast |
| Bone marrow and blood | |
| Bone morphogenetic protein receptor (BMPR) | Mesenchymal stem and progenitor cells |
| CD4 and CD8 | White blood cell (WBC) |
| CD34 | Hematopoietic stem cell (HSC), endothelial progenitor |
| CD34+Sca1+ Lin- profile | Mesencyhmal stem cell (MSC) |
| CD38 | Absent on HSC Present on WBC lineages |
| CD44 | Mesenchymal |
| c-Kit | HSC, MSC |
| Colony-forming unit (CFU) | HSC, MSC progenitor |
| Fibroblast colony-forming unit (CFU-F) | Bone marrow fibroblast |
| Hoechst dye | Absent on HSC |
| Leukocyte common antigen (CD45) | WBC |
| Lineage surface antigen (Lin) | HSC, MSC, Differentiated RBC and WBC lineages |
| Mac-1 | WBC |
| Muc-18 (CD146) | Bone marrow fibroblasts, endothelial |
| Stem cell antigen (Sca-1) | HSC, MSC |
| Stro-1 antigen | Stromal (mesenchymal) precursor cells, hematopoietic cells |
| Thy-1 | HSC, MSC |
| Cartilage | |
| Collagen types II and IV | Chondrocyte |
| Keratin | Keratinocyte |
| Sulfated proteoglycan | Chondrocyte |
| Nervous system | |
| CD133 | Neural stem cell, HSC |
| Glial fibrillary acidic protein (GFAP) | Astrocyte |
| Microtubule-associated protein-2 (MAP-2) | Neuron |
| Myelin basic protein (MPB) | Oligodendrocyte |
| Nestin | Neural progenitor |
| Neural tubulin | Neuron |
| Neurofilament (NF) | Neuron |
| Neurosphere | Embryoid body (EB), ES |
| Noggin | Neuron |
| O4 | Oligodendrocyte |
| O1 | Oligodendrocyte |
| Synaptophysin | Neuron |
| Tau | Neuron |
| Fat | |
| Adipocyte lipid-binding protein (ALBP) | Adipocyte |
| Fatty acid transporter (FAT) | Adipocyte |
| Adipocyte lipid-binding protein (ALBP) | Adipocyte |
| General | |
| Y chromosome | Male cells |
| Karyotype | Most cell types |
| Liver | |
| Albumin | Hepatocyte |
| B-1 integrin | Hepatocyte |
| Pancreas | |
| Cytokeratin 19 (CK19) | Pancreatic epithelium |
| Glucagon | Pancreatic islet |
| Insulin | Pancreatic islet |
| Insulin-promoting factor-1 (PDX-1) | Pancreatic islet |
| Nestin | Pancreatic progenitor |
| Pancreatic polypeptide | Pancreatic islet |
| Somatostatin | Pancreatic islet |
| Pluripotent stem cells | |
| Alkaline phosphatase | Embryonic stem (ES), embryonal carcinoma (EC) |
| Alpha-fetoprotein (AFP) | Endoderm |
| Bone morphogenetic protein-4 | Mesoderm |
| Brachyury | Mesoderm |
| Cluster designation 30 (CD30) | ES, EC |
| Cripto (TDGF-1) | ES, cardiomyocyte |
| GATA-4 gene | Endoderm |
| GCTM-2 | ES, EC |
| Genesis | ES, EC |
| Germ cell nuclear factor | ES, EC |
| Hepatocyte nuclear factor-4 (HNF-4) | Endoderm |
| Nestin | Ectoderm, neural and pancreatic progenitor |
| Neuronal cell-adhesion molecule (N-CAM) | Ectoderm |
| Oct-4 | ES, EC |
| Pax6 | Ectoderm |
| Pluripotent stem cells | |
| Stage-specific embryonic antigen-3 (SSEA-3) | ES, EC |
| Stage-specific embryonic antigen-4 (SSEA-4) | ES, EC |
| Stem cell factor (SCF or c-Kit ligand) | ES, EC, HSC, MSC |
| Telomerase | ES, EC |
| TRA-1-60 | ES, EC |
| TRA-1-81 | ES, EC |
| Vimentin | Ectoderm, neural and pancreatic progenitor |
| Skeletal muscle/Cardiac/Smooth muscle | |
| MyoD and Pax7 | Myoblast, myocyte |
| Myogenin and MR4 | Skeletal myocyte |
| Myosin heavy chain | Cardiomyocyte |
| Myosin light chain | Skeletal myocyte |
Table 1: Markers used to identify stem cells in differentiated cell types
Using a murine bone marrow stromal cell line, cell fractions have been expanded which express neither CD34 nor lineage markers (CD34- / Lin- cells) and converted to CD34+ cells. It has been observed that there is a common precursor cell of HSCs and MSCs giving rise to bone, cartilage, and other mesenchymal organ systems. Thus, CD34- Flk1+ cells, which have been identified as putative “hem angioblasts,” might, in fact, be more primitive than CD34+ / Flk 1+ cells. In fact, human Flk1+/ CD31- / CD34- cells could not only contribute to hematopoietic and vascular reconstitution, but they also readily give rise to epithelial cells of the liver, lung, and gastrointestinal tract of irradiated NOD / SCID mice [9]. No contribution was seen to skeletal, cardiac muscle, skin, and kidneys. It has been shown that these differences may be due to less damage induced by irradiation in these tissues or more residual tissue–specific stem cells capable of self-repair within these tissues. Differentiation of FLK1+ / CD31- / CD34 – cells into hematopoietic cells cannot be attributed to the contamination by hematopoietic stem/ progenitor cells. BM cells were depleted of CD45+ and CD34+ cells by magnetic bead sorting before Flk1+/ CD31- / CD34 – cell cultures were initiated. All input Flk1+ / CD31- /CD34- cells were CD11a, CD11b, GlyA, and CD45 negative and did not express early hematopoietic transcription factors, including GATA -1 and GATA-2. Additionally, culture conditions for Flk1+ / CD31- / CD34- cells are not supportive of hematopoietic stem/progenitor cell differentiation. Presently the trend is to select stem cells on the basis of their markers and provide adequate cell culture conditions so that the cells can differentiate in a lineage-specific manner. It has been shown that neural cells are differentiated in vitro from human embryonic stem cells (hESC) which show broad cellular heterogeneity with respect to developmental stage and lineage specification. They have shown standard conditions for the use and discovery of markers for analysis and cell selection of hESC undergoing neuronal differentiation. To generate better-defined cell populations, they established a working protocol for sorting heterogeneous hESC-derived neural cell populations by fluorescence-activated cell sorting (FACS). Using genetically labelled synapsin-GFP (+) hESC-derived neurons as a proof-of-principle they enriched viable differentiated neurons by FACS. Cell sorting methodology using surface markers was developed, and comprehensive profiling of surface antigens was obtained for immature ES cell types (such as SSEA-3, -4, TRA-1-81, TRA-1-60), neural stem and precursor cells (such as CD133, SSEA-1 [CD15], A2B5, FORSE-1, CD29, CD146, p75 [CD271]) and differentiated neurons (such as CD24 or NCAM [CD56]). At later stages of neural differentiation, the neural cell adhesion molecule NCAM (CD56) was used to isolate hESC-derived neurons by FACS. FACS-sorted hESC-derived neurons survived in vivo after transplantation into the rodent brain. These results show (a) a feasible approach for experimental cell sorting of differentiated neurons, (b) an initial survey of surface antigens present during neural differentiation of hESC, and (c) a framework for developing cell selection strategies for neural cell-based therapies. Further, it has been shown that stem cells with CD133 can follow bi-lineages if appropriate conditions are provided. Recent studies have shown new shreds of evidence that several tissues may contain cells capable of generating differentiated cells beyond their own tissue boundaries, defining a process termed stem cell plasticity. The type of cells which show plasticity are bone marrow cells which give rise to blood cells of all lineages, and mesenchymal stem cells which give rise to osteoblasts, adipocytes, and fibroblasts There are reports showing that bone marrow stem cells can evolve into cells of all dermal lineages, such as hepatocytes, skeletal myocytes, cardiomyocytes, neural, endothelial, epithelial, and even endocrine cells. These findings promise significant therapeutic implications for regenerative medicine.
It has been reported that epigenetic alterations sometimes cause the silencing of genes required for HSCs to undergo symmetrical cell divisions [10,11]. As a result of this, there is a progressive decline of primitive HSC function. It has been seen that the self-renewal capacity of HSCs decreases progressively with HSC differentiation. The mechanisms, which govern stem cell growth, are under tight control but still, these are modifiable. The global gene expression of HSC has been described but very little is known about the dynamics of gene expression necessary for HSC fate decisions. Oct-4 gene expression is shown to be regulated by an epigenetic mechanism, which is required for the maintenance of pluripotency of embryonic stem cells [12]. The extrinsic and intrinsic factors for the growth of HSCs are still not known. However, it is thought that growth factors and an adequate microenvironment are crucial for the survival and proliferation of HSC’s self-renewal/differentiation decisions in HSC appear likely to occur independently of cytokines and are postulated to be determined by the intrinsic properties of HSC [13]. HSC are positive for CD34 and all those cells, which carry the gene P15, are un methylated but after 7 days of culture, the cells undergo the process of methylation and demethylation after 15 days. HSCs can undergo either a symmetric or asymmetric, cell division. Under both conditions, the microenvironment should be such that there is the sustenance of long-term donor-derived haematopoiesis. The methylation machinery in normal hematopoietic development is regulated to allow lineage-specific differentiation and control of proliferation, disturbances in methylation lead to gene-specific inactivation by promoter silencing [14]. It has been suggested that in combination with demethylating agents with Histone deacetylase inhibitors (HDAC) can be used to remove the epigenetic gene inactivation [15]. It has been reported that bone marrow cells have the ability to evolve or differentiate into oral and craniofacial tissues, such as the periodontal ligament, alveolar bone, condyle, tooth, the bone around dental and facial implants, and oral mucosa [16].
If bone marrow cells could give rise to stem cells of another tissue, then they could repopulate whole organs from a few starting cells. This model of dedifferentiation is consistent with recent data from animal models. Genetic analysis of cells of donor origin in vivo and in vitro has brought to light another possible mechanism. The shreds of evidence have been gathered from tracking techniques as shown in (Figure 3).
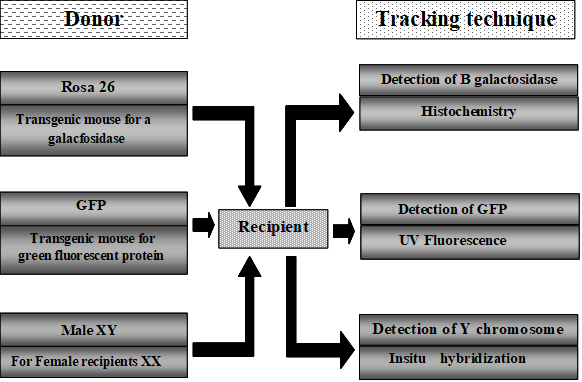
Figure 3: Methods to track the transplanted stem cell
The haematopoietic and neural stem cells appear to be most versatile at cutting across lineage boundaries and regenerative medicine [17]. Mesenchymal stem cells (MSCs) have regenerative capacity and can be obtained from mesenchymal tissues, such as bone marrow, adipose tissue, and the umbilical cord, and have trophic and immunosuppressive effects. MSCs can differentiate into cells of three germ layers.
MSCs belong to the mesodermal lineage, but they are known to cross boundaries from mesodermal to ectodermal and endodermal lineages and differentiate into a variety of cell types both in vitro and in vivo. However, the scientific basis for the broad multipotent differentiation of MSCs still remains an enigma.
Cell fusion has emerged as a powerful subject of debate in the last few years [18]. Adult stem cell plasticity and the search for mechanisms to explain this process have led to the "rediscovery" of cell fusion (Figure 4).
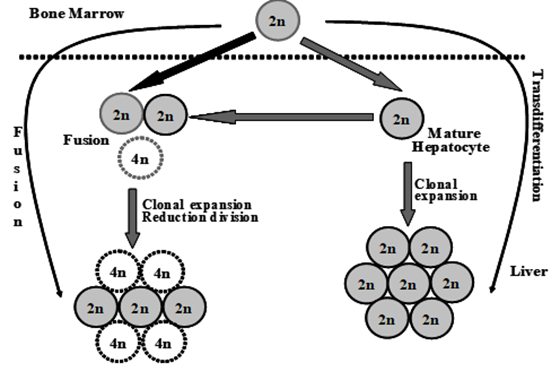
Figure 4: Differentiation stages of HSCs into hepatocytes
Differentiation of HSCs into hepatocytes has shown that trans-differentiation takes place upon exposure to the hepatic environment, donor HSCs undergo genetic reprogramming, switch lineage, and generate hepatocytes. The frequency at which this occurs depends on several factors, including the type and extent of liver injury. If the fusion of donor HSCs, or indeed other hematopoietic cells such as macrophages, fuse with mature hepatocytes these eventually re-differentiate into terminally differentiated hepatocytes. It should be noted that although most fusion cells contain multiples of the normal karyotypes, approximately 30% of cells are aneuploid.
Donor HSCs engraft and differentiate into hepatocytes, which then undergo fusion with mature native hepatocytes. All these steps are shown in Figure 5.
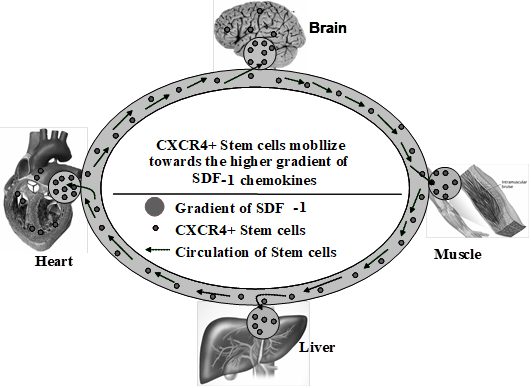
Figure 5: Role of CXCR4 and stem cell in different organs
Mobilization of haematopoietic stem cells Stem cell mobilization is an important phenomenon both in bone marrow transplantation and stem cell therapy. It has been proposed that for mobilization various molecules like adhesion molecules e.g. selectins and integrins, chemokines and their ligands e.g. SDF 1α, CXCR4 and proteolytic enzymes are required (Figure 6).
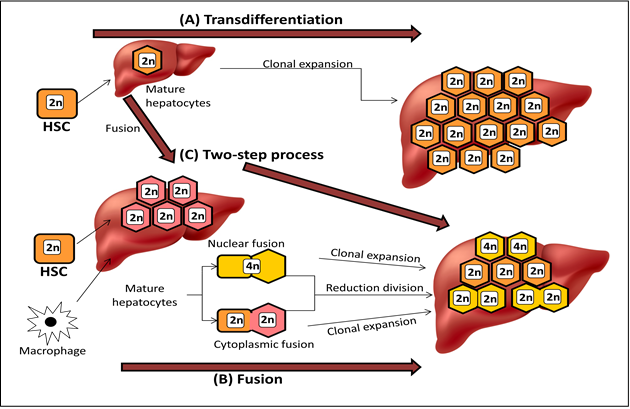
Figure 6: Mobilization of hematopoietic stem cells.
CXCR4 circulate at low levels in the peripheral blood. The concentration increases during tissue damage. The chemo-attractants released from the damaged tissue like SDF-1 lead the stem cells to ‘home in’ at the site of injury.
Mobilization is important because it protects HSC from toxic injuries. Sometimes mobilization could be a death pathway a mechanism that can regulate self-cell number. Mobilization mechanisms may be complementing asymmetrical division [19]. Under mobilization, if a replication event occurs and the microenvironment for the cell is not available, one of the cells will mobilize or die. The mobilization of stem cells depends on SDF-1α / CXCR4 and VLA-4 / VCAM-1 pathway. VLA-4 / VCAM-1 pathway is important for the housing of bone marrow stem cells [20].
It has been demonstrated that CD34+ are involved in lodging in both bone marrow and spleen [21]. It has been demonstrated that entry of CFU (colony-forming units) in the spleen is highly regulated. Further VLA-4 and CD44 are identified in the mobilization of stem cells into the bloodstream of mice when injected with anti-VLA4 or anti-CD44 [22]. Stem cells mobilized by anti-VLA 4 comprise high self-renewal potential and can be used for long-term reconstitution of tissue [23]. It has been shown that bone marrow contains HSC and mesenchymal stem cells (MSCs) both of which may be derived from a common primitive blast-like cell precursor which is able to differentiate along MSC or HSC potentials. HSCs return to the bone marrow within 1 day. Different factors are involved in the migration and homing of the ligand for C kit, B1 integrins, chemokine stromal-derived factors (SDF) or its receptors. CXCR4 prevents haematopoiesis [24]. from transferring from the embryonic liver to the marrow. SDF1 seems to be a chemoattractant for HSC expressing CXCR4 [25]. Although CXCR4 cells may also migrate towards SDF1 and it has been demonstrated in vivo that CXCR4 expression on CD34+ CD38- Lin- cells does not confer any advantage in the rescue of irradiated NOD/ SCID mice. Colony stimulating factor helps in mobilization. In wound healing, the stem cells sense tissue damage and migrate from the distance to the site of injury. Nevertheless, it has been observed that activation of the immune response increases the regeneration of cells and tissues when transplanted to the host. There is a link between blood and endothelial cell lining in the form of a multipotent stem cell, haem angioblast, which gives rise to both haematopoietic and endothelial cell lines. This is a debatable issue but the finding that a cell, such as a monocyte, which is presumed to be fully committed to myeloid bloodlines, is also capable of generating endothelial cells, has significant consequences for stem cell biology and innate immunity [26].
During autologous stem cell transplantation, the source could be bone marrow or peripheral blood as the source of stem cells. Normally collection of stem cells from PBSC is difficult and has fewer stem cells compared to bone marrow. But in case of damaged bone marrow or heavy tumour infiltration, PBMCs remain the only option. In these situations, various mobilization regimens can be used to increase the CD34+ concentration in the peripheral blood and thus more CD34+ cells than steady-state bone marrow could be obtained [27]. The infusion of more CD34+ cells not only results in more rapid engraftment but also in a lower risk of being contaminated with tumour cells residing in the bone marrow. However, in allogeneic stem-cell transplantation, the engraftment is faster with PBMSC than with bone marrow thus negating the number of infused CD34+ cells as a critical factor. Recently an interesting report has been published showing that genome-editing technologies have the potential to correct the SCD (Sickle Cell Disease) mutation in hematopoietic stem cells (HSCs), producing adult haemoglobin while simultaneously eliminating sickle haemoglobin. The authors have developed high-efficiency viral vector-free non-footprint gene correction in SCD CD34+ cells with electroporation to deliver SCD mutation-targeting guide RNA, Cas9 endonuclease, and 100-mer single-strand donor DNA encoding intact β-globin sequence, achieving therapeutic-level gene correction at DNA (∼30%) and protein (∼80%) levels. Gene-edited SCD CD34+ cells were corrected cells 6 months’ post-xenograft mouse transplant without off-target δ-globin editing. Afterwards, they developed a rhesus β-to-βs-globin gene conversion strategy to model HSC-targeted genome editing for SCD and demonstrated the engraftment of gene-edited CD34+ cells 10-12 months post-transplant in rhesus macaques [28]. Another concern in allogeneic transplantation is graft-versus-host disease (GvHD), caused by lymphocyte subpopulations, particularly T-lymphocytes, thus limiting the use of donor PBSC as the stem-cell graft in allogeneic transplantation. However, instead of steady-state PBMCs, mobilized PBSC from T lymphocytes depleted bone marrow are used for allergenic stem-cell grafting, and acute GvHD is less frequent (29). There are studies which have shown that for good-risk patients (HLA-identical related donor; low or average risk of relapse) bone marrow and peripheral blood are equivalent. However, it has been demonstrated that for good-risk younger patients, the bone marrow provides better results. Donor preference is also a critical factor and is the choice of stem cells to match unrelated donor transplants. For allogeneic SCT from partially matched related donors, the number of CD34+ cells infused is very critical. As a higher number of purified CD34+ cells can only be obtained from PBSCs instead of bone marrow hence the use of PBSC appears as a better choice for allogeneic HSCT. Umbilical cord blood as the source of HSCs Umbilical cord blood provides an optional therapeutic modality for hematopoietic stem cell therapy. However, it has the limitation of fewer numbers of HSCs thus delaying the post-transplant engraftment. One of the options to overcome this limitation is selective ex vivo expansion of primitive progenitor stem cells. A better understanding of the microenvironment of haematopoiesis enabled the expansion of these cells without their differentiation. However, it has been reported that cellular defects may be acquired during ex vivo expansion. This may be due to defects in the cell cycle abnormalities. It has been demonstrated that once the cells enter the G1 phase and are exposed to cytokine these cells reveal reduced engraftment capacity. CD34+ cells cultured in the presence of FL, TPO, and SCF show increased adhesion hence decreased marrow homing. Further, if the cells are exposed to IL3, IL6, SCF, IL11 and FL there is an altered expression of VLA -4 and VLA-5, which causes poor engraftment. Ex vivo cultures are associated with increased expression of the Fas ligand (CD95), this downregulates the expression of the anti-apoptosis protein Bct-2 and increased caspase activation resulting in the induction of apoptosis. Cell differentiation is due to cell fusion. Stem cells may undergo fusion with other cell types. Recent development in the optimization of HSC culture has revealed the choice of cell source, cytokines and stroma used in culture could affect the success of the specific expansion of stem cells. The increasing interest has grown in the molecular mechanism that regulates the specific expansion in ex vivo. There are various signalling pathways involved in HSC proliferation and maintenance.
Some examples of these pathways are: Notch signalling pathway: Notch signalling is involved in cellular differentiation, proliferation, apoptosis, adhesion and epithelial–to–mesenchymal transition.
There are five Notch ligands in mammals, Jagged -1/2 and Delta – 1/3/4. The Notch ligand binds to a Notch receptor which results in the cleavage and causes the release of the intra- cellular domain of the Notch receptor by a membrane–associated protease complex. The intracellular domain then translocates to the nucleus to join with CBF1/ RBP-J and mastermind–like (MAML) among others. The assembled nuclear complex regulates the transcription of several Notch effector genes, including a homolog of Drosophila. Thus the optimal concentration of Notch ligands in ex – vivo culture has yet to be established and the differing effects of Notch ligands have not yet been fully studied; for instance, Delta-1, unlike Jagged-1, inhibits B – cell differentiation and decreases CFU-GM, CFU-G and CFU-M. Wnt signalling pathway: Wnt-mediated signalling involves the binding of Wnt proteins to their receptor-co receptor complexes, frizzled- LRP, which leads to the accumulation of β-catenin and its translocation to the nucleus. Early studies demonstrated that activation of Wnt signalling induced both mouse fetal liver HSC (AA4+ cKIT+ SCA1) and human bone marrow (Lin)-CD34+ proliferation when HSC were co-cultured on Wnt transduced stromal feeder layers. Interestingly, Wnt-mediated maintenance of HSC immaturity in vitro requires Notch signalling, although HSC survival and entry into the cell cycle is Notch independent. Tie2/ Ag-1 signalling pathway: Tie2 /Ang-1 signalling pathway plays a critical role in the maintenance of HSC in a quiescent state in the BM niche. BMP: BMP is a member of the TGF-β superfamily that has long been established to function in the development and regulation of a wide range of biological systems. BMP also play a key role in regulating fate choices during stem cell differentiation. The BMP receptor activation of spindle-shaped N-cadherin +CD 45- osteoblastic type (SNO) cells maintain the stem cell niche. Bmi-1: B lymphoma Mo-MLV insertion region 1 (Bmi-1), a proto-oncogene and member of the Polycomb group repressive complex 1. The importance of Bmi -1 in HSC self-renewal has been demonstrated in mouse models of both genetic deletion and transduced over expression. As a central player in HSC self–renewal, Bmi-1 could be a target for the therapeutic manipulation of HSC. HOXB4: HOX genes encode a large family of transcription factors that have a highly conserved DNA –binding motif known as the homeodomain.
HOXB4 may be one of the most important regulators of HSC self-renewal. It is expressed in the stem cell fraction of the bone marrow and subsequently down-regulated during differentiation in humans and mice. HOXB4–deficient mice have HSC with a reduced proliferative capacity but normal differentiation and lineage pathways forced expression of HOXB4 also rapidly triggers an increase of human UCB HSC detected by both in vitro and in vivo assays. It has been shown that improvement in the culture systems may expand UCB progenitors. Some of these manipulations are summarized in (Table 2).
| Target | Description |
| Epigenetic modification | Epigenetic modification of growth factors by histone methylation inhibition or acetylation (5azaD/ TSA) may slow differentiation. |
| Cell types | CD34+cells, however, cells with more primitive marker CD133+ provides better engraftment and more accurate functional assays. |
| Copper chelation | Intracellular copper concentration may be crucial for an immature phenotype and then the engraftment. |
| Cytokines | SCF, Flt-3 ligand (FL) and thrombopoietin (TPO) are crucial cytokines. FL and TPO may prevent apoptosis. |
| Retinoids | These are involved in the embryonic development and cellular differentiation. Success has been achieved in using all-trans retinoic acid to increase murine long-term repopulating cells. |
| Wnt activation | The Wnt pathway is involved in the maintainance of the undifferentiated phenotype in human ebryonic stem cells. Use of Wnt activator to HSC recipient mice improved better recovery. |
Table 2: Manipulations in the UCB ex vivo expansion
With a better understanding of the molecular mechanisms of HSC homing, expansion and presentation, a second generation of clinical trials is being initiated and much is based on the enhanced understanding of hematopoiesis his is occurring at the same time that other clinical trials explore the use of dual UCB units as the transplant source. Although cord transplants may ultimately supplement our need for HSC expansion, it remains to be determined if this approach will relapse and optimize immune reconstitution as compared to that seen after a living-related or unrelated donor transplant.
Micro RNA expression in stem cells
It has been reported that micro–RNA is expressed specifically dividing embryonic development hence it is thought that their role may be critical for stem cell differentiation and cell proliferation, especially at the level of gene regulation. Recently a miRNA microarray technique has helped to study developmental biology but tissue homeostasis and malignant cell proliferation are still poorly understood. Their role is also demonstrated in model organisms like Drosophila, Dicer-1 (DCR-1) which is essential for the formation of mature RNA which is when mutated in Drosophila germline stem cells (GSC) they could not be maintained, suggesting their role is GSC self-renewal. Their mode of action is hypothesized to regulate various target cells involved in cell differentiation and proliferation.
Stem cell therapy
Experimental biology and medicine have used stem cells in cell therapy for more than 20 years. An in vitro method has been developed to culture embryonic stem (EC) cells acquired at abortions or from surplus embryos left after in vitro fertilizations, and immediately evoked ideas on how to direct the development and differentiation of these cells and utilize them in the regeneration of damaged tissues. Still, cell therapy faces the difficult task how to detect, harvesting and culturing stem cells for the treatment of several diseases.
CD34+ and FLK-1+ stem cells can differentiate into endothelial cells in vivo and in vitro. These can be helpful in ischemic injury. These cells get mobilized after ischemia or after G-CSF pre- treatment. Homing of HSCs has been reported in the lungs, gastrointestinal tract, liver, pancreas, kidney, skin, skeletal muscle and bone. HSCs treatment has been successfully explored in various diseases (Table 3).
| Autologous transplantation | Allogeneic transplantation |
| Multiple myeloma | Multiple myeloma |
| Non-Hodgkin’s lymphoma | Non-Hodgkin’s lymphoma |
| Hodgkin’s disease | Hodgkin’s disease |
| Acute myeloid leukemia | Acute myeloid leukemia |
| Neuroblastoma | Chronic lymphocytic leukemia |
| Ovarian cancer | Juvenile chronic myeloid leukemia |
| Germ-cell tumors | Acute lymphoblastic leukemia |
| Autoimmune disorders | Chronic myeloid leukemia |
| Amyloidosis | Myelodysplastic syndromes Aplastic anemia |
| Blackfan–Diamond anemia | |
| Thalassemia major | |
| Sickle cell anemia | |
| Severe combined immunodeficiency | |
| Inborn errors of metabolism |
Table 3: Diseases treated with hematopoietic stem-cell transplantation
Some studies show that transplantation of pluripotent stem cells or foetal cells can successfully treat a number of chronic diseases, such as diabetes, Parkinson’s disease, traumatic spinal cord injuries, Purkinje’s cellular degeneration, liver failure, heart failure, Duchene’s muscular dystrophy, ontogenesis imperfecta, and others (30). Although marked progress has been achieved in human transplant therapy, there are still several main setbacks limiting the broad application of cells in routine therapy, such as the need for massive doses of immunosuppressive drugs to prevent the rejection of transplanted tissue and also the lack of organs from dead donors. Despite all these setbacks, a strategy based on human ES cells may allow the production of unlimited amounts of cells, eventually tissues, and their sufficient supply from abundant, renewable and quickly available sources. Moreover, ES cells according to their adaptability for stable genetic modification could be treated so as to avoid or inhibit the host immune response. The first step to developing successful therapy based on human ES cells is to demonstrate their capability to differentiate into certain, cell types, and to purify this line from a mixed population. In the second step, it would be necessary to critically examine that differentiated cell derivatives function in a normal physiologic way, for example, the secretion of insulin in the cells of Langerhans islets is normal and responds to the glucose level. The third step and most important milestone on the route to clinical tests will be the proof of the efficiency of model diseases in guinea pigs and big animals. The fourth step is to exclude the formation of tumours developing from derivatives after differentiation of ES cells and transplanted to human recipients. Considering the progress in these directions goes forward in a big way, however, other problems will certainly show which may limit the therapeutic use of cells. The effort of scientists to treat diseases at present untreatable, the pressure of patients and their families, as well as political pressure may complicate the development of new therapies. Important is to keep a “clear mind”, get rid of emotions and respect scientific and ethical rules. Prospective trends in cell therapy are therapeutic cloning, ES cells, therapy of the foetus, adult stem cells, use of humoral agents for control of stem cell behaviour and, eventually, genetic stem cell modification. In the beginning, it appeared that adult stem cells may represent a certain “ethical compromise” to embryonic cells. Today, however, we understand that individual approaches are closely linked together and this, consequently, delays the answers to bioethical issues. Scientists have already shown that a number of cell types, such as neurons and muscle cells, pancreatic cells and others can be obtained by culture of ES cells. Today, stem cells may be used in quite unexpected cases, such as renal diseases and immunologic repair in AIDS patients. During the past three decades, allogeneic stem cell transplantation (ASCT) has developed from being an experimental therapy in patients with end-stage leukaemia into a well-established therapy in patients with a range of disorders of the immune hematopoietic system.
Graft-versus-host disease (GVHD), acute or chronic, violent host tissue is a key threat. However, donor immunocompetent T cells have a potent graft-versus-leukaemia effect. The calcineurin inhibitors and methotrexate combination is the standard therapy to prevent GVHD. Intonation of the immunosuppressive regimen may persuade mild acute and mild chronic GVHD, reduce the risk of relapse, and improve long-term survival. Natural killer cells also play a role in this context. Killer cell immunoglobulin-like receptor incompatibility between recipient and donor may reduce the risk of relapse in patients with myeloid leukaemia. Relapse of leukaemia is a major cause of death after ASCT. Minimal residual disease and recipient leukaemia lineage-specific chimerism are sensitive techniques for early detection of leukemic relapse. Donor lymphocyte infusions can enhance the antitumor effect, especially for patients with molecular relapse. The allogeneic graft-versus-cancer effect has been demonstrated in patients with metastatic breast, renal, colorectal, ovarian, prostatic, and pancreatic carcinoma. Mesenchymal stem cells have immunomodulatory properties and may be used for the immunomodulation of GVHD and tissue repair. If all modalities are considered, the future looks promising for ASCT.
Autoimmune diseases
Autoimmune diseases vary in a wide range from mild to severe, intractable diseases. A great development of the therapy has been encountered in the past decades, in particular of formerly incapacitating diseases, such as rheumatoid arthritis or Crohn's disease. While biological therapy, in particular monoclonal antibodies, offered most new solutions for these disorders, the therapy with many conventional drugs has also been rewritten. Old drugs, such as cyclophosphamide or intravenous immunoglobulin, retained their position in severe forms of autoimmunity. An evolving new area of stem cell transplantation offers benefits for the most severe patients suffering from intractable autoimmune diseases. ESCs under controlled environments can be differentiated into different cell lineages like cartilage and bone. Cultured articular chondrocytes, MSCs have been used for cartilage repair [31]. However, the results are inconsistent. This is because many other factors are also involved like growth factors, tissue scaffolds, diseased microenvironment and alignment of the joint. These are some of the crucial conditions responsible for success. Presently osteoinductive growth factors that derive MSCs down the osteogenic pathways are under the process of trial in different clinical settings. However, the osteogenic pathway may not be responsible but other factors relating to the vascularization of bones may be responsible. Stem cell therapy has also been used to repair damaged muscles. However, the success rate is not very high.
In Osteoarthritis (OA) stem cells have been tried and it has been shown that they are involved in the remodelling process including osteophytosis. Most of the time the failure of MSCs therapy is due to inflammatory cytokines which show detrimental effects on bones and cartilage. From these results, it appears that the diseased microenvironment playsan important role in the functioning of osteoarthritis. It is proposed that for degenerative therapy in musculoskeletal diseases, an artificial microenvironment may be created which is capable of supporting cell engraftment, committing differentiation, resulting in patterning and maturation of tissue, subsequent anatomy and function. Presently self-assembling in structuring polymers are being used which controls the release of bioactive substances oblique morphogens. These polymers help in cell adherence, proliferation and differentiation as well as biomechanical support. Adipose tissue-derived stromal cells (ADSC) can easily be isolated from human adipose tissue and they have the potential to differentiate into bone, cartilage, tendons, skeletal muscle, and fat when cultivated under lineage-specific conditions. Tissue engineering of these mesenchymal organs is of major interest in human diseases, such as inherited, traumatic, or degenerative bone, joint, and soft tissue defects (skeletal regeneration and cartilage repair). It has been proposed that adipose tissue-derived stromal cells (ADSC) have an equal potential to differentiate into cells and tissues of mesodermal origin, such as adipocytes, cartilage, bone, and skeletal muscle. HLA and haploidentical HSCT HLA plays an important role in identifying self from non-self, which is very important in transplant biology. HLA proteins on the outer surfaces of most cells of the body are inherited from our parents and are located on chromosome 6. These antigens are inherited in a co-dominant fashion. In the case of BMT, donor selection is either from the family or from an unrelated marrow search. When no matched sibling or unrelated donor exists, the potential curative option is haplo-HSCT, that is, transplant with a donor who shares only one haplotype with the recipient. Haploidentical stem cell transplants are increasingly used in the treatment of malignancies, and immune and hematologic diseases. As multiple mismatched related donors may be available for transplantation, it is important to select a donor that is most likely to produce a successful outcome. There are studies that correlate the HLA-B mismatch effect in haplo-HSCT. Studies analysed the impact of HLA-A, -B, -DRB1, -DRB3, -DRB4 and -DRB5 and demonstrated that an HLA-B mismatch not only has a significant effect on GVHD and transplant-related mortality but was also associated with reduced OS and leukaemia-free-survival There is an important point in haploidentical transplants that should be considered: the conditioning regimen. Many protocols have been performed to improve the outcomes of transplantation and to minimize the effect of HLA incompatibility. For example, studies on non-myeloablative HLA-haploidentical bone marrow transplantation with high-dose post-transplant cyclophosphamide. The results showed that HLA mismatch was not associated with relapse or GVHD. In bone marrow transplantation with the mismatch of the HLA-DRB1 antigen in the GVHD direction and two or more HLA Class I (HLA-A, -B and -Cw) mismatches in either direction were found to be associated with decreased incidences of relapse without an increased incidence in non-myeloablative conditioning with post-transplant cyclophosphamide HLA and cord blood transplantation The use of umbilical cord blood transplantation (UCBT) for patients with haematological malignancies or hereditary diseases is becoming increasingly more common. Hence, studies on the distribution of HLA alleles and haplotypes in different ethnic populations are also important to find a suitable unrelated cord blood donor for a patient. Presently importance has been given in the case of BMT to human natural killer (NK) cells which are components of the innate immune response that comprise approximately 10-15% of all peripheral blood lymphocytes and play a major role in immunity against viral infections and tumours Years of intensive research in mice and humans have shown special importance of NK cells in the haematological diseases and in mediating favourable HSCT outcomes. The mechanism of recognition of a target cell by NK cells differs from others lymphocytes. The NK cells are able to recognize a reduction or absence of self HLA class I ligands, as a form of distinguishing normal cells from target cells: this is the “missing-self recognition”. It is well established that cancer cells and some infected cells develop various mechanisms to escape lysis by T cells. An effective mechanism is to decrease or remove completely the HLA expression. The downregulation of HLA class I expression leads to resistance to lysis by T lymphocytes but, as a consequence, can lead to a susceptibility to lysis by NK cells. During development, NK cells become licensed or educated by interaction with self-HLA molecules to maintain tolerance to normal tissues. NK cells that do not express inhibitory receptors for self are retained in an anergic or hypofunctional state and those which express inhibitory KIRs for self-HLA ligands are functionally active and thus can sense the lack of expression of self HLA molecules on target cells which trigger lysis of these cells. This is thought to be the main mode of action of NK cells [32].
Natural killer cell alloreactivity in hematopoietic stem cell transplantation
The clinical significance of missing-self recognition is especially evident in allogeneic HSCT (33). In HSCT the NK cell alloreactivity is determined by an analysis of the donor’s KIR gene profile and by differences in MHC class I genes between the donor and the recipient (34). This can be better explained by the presence in the donor of NK cells expressing inhibitory KIRs that are not engaged by any of the HLA class I alleles present on the receptor As a consequence, donor NK cells become uninhibited and may display alloreactivity against mismatched allogeneic targets. Furthermore, NK cells are relevant in the setting of HSCT because they are the first lymphoid cell subset to reconstitute after transplantation at a time when the adaptive immune system is impaired. Additional approaches in which NK cells may represent an important tool for cancer therapy, are to exploit the unique properties of the "adaptive" NK cells. These CD57+ NKG2C+ cells, despite their mature stage and potent cytolytic activity, maintain a strong proliferating capacity. This property was revealed to be crucial in hematopoietic stem cell transplantation (HSCT), particularly in the haplo-HSCT setting, to cure high-risk leukaemia. T-depleted haplo-HSCT (e.g. from one of the parents) allowed for saving the life of thousands of patients lacking an HLA-compatible donor. In this setting, NK cells have been shown to play an essential role against leukaemia cells and infections. Another major advance is represented by chimeric antigen receptor (CAR)-engineered NK cells. CAR-NK, different from CAR-T cells, may be obtained from allogeneic donors since they do not cause GvHD. Accordingly, they may represent "off-the-shelf" products to promptly treat tumour patients, at affordable costs. Different from NK cells, helper ILC (ILC1, ILC2 and ILC3), the innate counterpart of T helper cell subsets, remain rather ambiguous with respect to their anti-tumour activity. A possible exception is represented by a subset of ILC3: their frequency in peri-tumoral tissues in patients with NSCLC directly correlates with a better prognosis, possibly reflecting their ability to contribute to the organization of tertiary lymphoid structures, an important site of T cell-mediated anti-tumour responses. It is conceivable that innate immunity may significantly contribute to the major advances that immunotherapy has ensured and will continue to ensure to the cure of cancer [35].
KIR model studies
Considering: 1) a strong correlation between the presence of KIR genes and their HLA ligands and cytotoxicity and 2) the advent of methods of precise genetic characterization, it is possible to determine the contributions of the various inhibitory and activating KIR genes in HSCT There are several models to define NK allure activity by KIR incompatibility or KIR mismatching, most of which are based on the analysis of KIR and HLA class I alleles. In the ligand model, the KIR expression is assumed following HLA typing. In this model, KIR ligands in recipients and donors are analyzed and at least one group of donor KIR ligands must be absent in the recipient’s KIR ligand repertoire. In the receptor-ligand model, the KIR genes are typed for the donor and the HLA alleles are analyzed for recipients and at least one of the inhibitory KIRs of the donor is not engaged in the recipient’s ligand repertoire. Moreover, some studies perform phenotypic analysis of inhibitory KIRs and CD94/NKG2A in donor NK cells and also functional assays which can provide more information about the degree of alloreactivity of NK cells It is difficult to know which model is the most adequate to select the KIR mismatch donor. Some authors suggest that an increasing number of receptor-ligand mismatch pairs increase the potency of the anti-leukaemia effect and also suggest that the receptor-ligand model could improve the accuracy of the prediction of relapse rather than the ligand-ligand model in patients with lymphoid malignancies However, it has not been well established and further studies are needed to confirm this hypothesis [36].
In addition, a novel observation emerged that NK cells of maternal donors of HSCT provided better protection from leukaemia relapse than other related donors. According to the authors, the better outcome of mother-to-child transplantation may be the result of the contact of maternal immune cells with the semi-allogeneic placenta during pregnancy. It was suggested that if further studies confirm the better outcomes of mother donors, it may be incorporated as a donor selection criterion.
Another interesting aspect was shown in a recent study with patients that received unrelated unmanipulated peripheral blood progenitor cells. The authors indicate that four-digit allele matching of HLA-C may have effects on the HSCT outcome dependent on the presence of C1 and C2 KIR epitopes in the patients suggesting the importance of analysis of HLA-C at the allele level for donor selection. While there are no common rules to select the best donor according to KIR compatibilities, all the findings must be analyzed [37].
KIR genes and haploidentical hematopoietic stem cell transplantation
Full-haplotype mismatched (haploidentical) HSCT is an option to treat patients lacking a matched donor or a suitable UCB unit. In haploidentical HSCT (haploid-HSCT), the T cells present on allogeneic hematopoietic grafts are important to promote engraftment and mediate the GvL effect. However, they can also mediate GVHD These T-cell responses can be controlled by an appropriate intensity of immunosuppression by the conditioning regimen. The T-cell depletion of the graft helps prevent GVHD but, as a consequence, T-cell depleted haplo-HSCT increases the risk of graft rejection and leukemic relapse. In this context, the presence of NK cell alloreactivity in the GVH direction seems to influence the prevention of leukaemia relapse and has been investigated in several preclinical and clinical trials. It has been observed that KIR-HLA mismatches can promote clinical benefits in haplo-HSCT, especially in patients with acute myeloid leukaemia (AML). KIR-Ligand incompatibilities were associated with a reduction in the risk of relapse of leukaemia and graft rejection, and also protection against GVHD in patients with AML. These results were supported by animal models, in which the presence of NK alloreactivity was suggestive of a low incidence of acute GVHD due to the killing of host APCs, which are critical for inducing donor T-cell activation Similarly, experimental data suggest that the engraftment rate was improved as a result of the lysis of residual host T lymphocytes by alloreactive donor NK cells and also that this contributed to the eradication of leukaemia blasts that escaped from the conditioning regimen. These studies showed very good results and led to a novel concept of mismatch to search for a transplant donor. Since then, several investigations based on KIR mismatching have been carried out with different outcomes.
The KIRs interact with some HLA class I antigens on target cells. HLA-Bw4 and distinct allotypes of HLA-C (C1 and C2 groups) are the main ligands for most KIRs. HLA-C alleles are classified as C1 or C2 KIR ligand groups, depending on two amino acid positions encoded in exon 2. HLA-C1 allotypes have serine at position 77 and asparagine at position 80 and are ligands for the KIR2DL2 and KIR2DL3 inhibitory receptors. HLA-C2 allotypes have asparagine and lysine at positions 77 and 80, respectively and are ligands for the KIR2DL1 inhibitory receptor and thought to be the ligand for the KIR2DS1 activating receptor HLA-Bw4 allotypes are characterized by at least 5 different patterns of amino acids at positions 77 and 80-83 and are ligands for KIR3DL1. Some HLA-A alleles, namely 23:01, 24:02 and 32:01, are also ligands for KIR3DL1. In addition, HLA-A3 and HLA-A11 are ligands for KIR3DL2; and HLA-A11 and some C1 and C2 allotypes are ligands for KIR2DS4. The KIR gene and respective ligands are listed in (Table 4).
| KIR | Function | Ligand |
| KIR2DL1 | Inhibitory | HLA-C group 2 |
| KIR2DL2 | Inhibitory | HLA-C group 1 |
| KIR2DL3 | Inhibitory | HLA-C group 1 |
| KIR2DL4 | Inhibitory, activating | HLA-G |
| KIR2DL5 | Inhibitory | Unknown |
| KIR3DL1 | Inhibitory | HLA-B Bw4 and some HLA-A Bw4* |
| KIR3DL2 | Inhibitory | HLA-A3 and HLA-A11 |
| KIR2DS1 | Activating | HLA-C group 2 |
| KIR2DS2 | Activating | Unknown |
| KIR2DS3 | Activating | Unknown |
| KIR2DS4 | Activating | HLA-A11 and subsets of HLA-C group 1 and group 2 |
| KIR2DS5 | Activating | Unknown |
| KIR3DS1 | Activating | Unknown |
* HLA-A*23:01, HLA-A*24:02 and HLA-A*32:01
Table 4: KIR receptors and their HLA ligands
KIR genes and autologous stem cell transplantation
Few research groups have demonstrated the influence of KIR genes in autologous stem cell transplantation (ASCT). The interest in the role of KIR genes in the setting of ASCT is mainly related to preventing relapse, the main cause of treatment failure. Some studies have shown that rapid and early NK cell recovery following ASCT is associated with better progression-free survival (PFS) in some diseases.
KIR genes and unrelated umbilical cord blood transplantation
Unrelated UCBT has proved to be a viable treatment option. An advantage of using UCB is the relatively low risk of acute GVHD due to a lower number of mature donor T cells and thus an increased possibility of using HLA-mismatched units. Moreover, UCBT, as in haplo-HSCT, is characterized by a rapid post-transplant recovery of NK cells.
Cytokines genes and receptors in HSCT
There are many other genetic factors that influence to outcome of a transplant, independent of whether the transplant is autologous, allogeneic, matched or mismatched, sibling or unrelated donor, or haploidentical and of whether the cell source is bone marrow, peripheral blood or UCB. The goal of the majority of studies is to know what kind of influence these genetic factors and HLA compatibility have and what effect they have on the course of the transplant: acute and chronic GVHD, relapse, OS and mortality. One important factor is the polymorphisms within the regulatory sequences of cytokine genes. Proinflammatory cytokines, receptors and related inhibitors have been implicated in a large number of immune diseases. The main role of cytokine genes is related to the immunopathogenesis of GVHD. Studies on cytokine genes in the transplant setting involve receptors of the TNF, IL-10, the IL-1 gene family, IL-2, IL-6, interferon TNF-γ, TGF-β1 and TGF-β1. Tissue injury, including the mucosa and liver, occurs during the conditioning regimen. This process causes the secretion of the TNF-α, IL-6 and IL-1 pro-inflammatory cytokines that increase HLA antigens, thus increasing the antigens recognized by donor T-cell receptors in allogeneic transplantation. Moreover, during the activation of donor cells, T cells produce IL-2 and INF-γ (Th1) that trigger GVHD and are balanced for Th2 cytokines such as IL-4 and IL-10 [38].
Major histocompatibility complex class I-related chain genes and HSCT
The MHC class I-related chain (MIC) genes have been the subject of interest in the setting of HSCT. This family of genes, located in the MHC classical class I region, was first described in 1994. These genes are very polymorphic, but not as much as the classical HLA class I genes. Humans have seven MIC genes, named MICA to MICG but only two MIC genes are functional, the MHC class I-related chain A (MICA) and B (MICB) genes. The MIC proteins are similar to the HLA class I gene products however they are not associated with β-2-microglobulin and also do not bind peptides to present to T cells. MIC proteins appear to be induced by stress and are expressed on the cell surface of fibroblasts and endothelium cells. They are ligands for NKG2D, a receptor present in NK cells and some T cells, and because of this they can co-stimulate NK cells and T cells and can therefore determine the outcome of certain effector functions that are related to GVHD. In fact, MIC genes have been shown to be attractive targets in diverse cancers, autoimmune diseases and organ rejection after transplantation.
Several studies have demonstrated that MICA may be a target molecule in allograft rejection because MICA can elicit antibody production after solid organ transplantation. Some studies have reported diverse outcomes in HSCT related to MIC genes. It was suggested that MIC genes play a role in GVHD in HLA-matched HSCT because a higher rate of grade II-IV acute GVHD was found as was more gastrointestinal GVHD in MICA mismatched patients. Some polymorphisms in MICA genes have also been associated with the outcomes in transplants [39]. A change at position 129 of the α2-heavy chain domain of MICA can denote the strength of interaction with the NKG2D receptor [40]. The presence of methionine at position 129 of the MICA gene characterizes a strong binder, and the presence of valine characterizes a weak binder. Hence, the MICA-129 valine genotype and soluble MICA serum level were considered risk factors for chronic GVHD in a study of 211 HLA-identical sibling pairs of HSCT while before transplantation, the presence of anti-MICA antibodies that can neutralize soluble MICA confers protection. Altogether, these data suggest that MIC genes, in particular the MICA genes, could be used as biomarkers for chronic GVHD and should be studied further [41].
Minor histocompatibility antigens and HSCT
The human minor histocompatibility antigens (mHAgs) are another group of immunogenic peptides, distinct from the MHC system, which seems to have a role in HSCT outcomes [42]. They are derived from intracellular polymorphic proteins and are presented by HLA class I and II-restricted T cells. Accumulated evidence suggests that they can elicit allogeneic T-cell mediated immune response in HLA-matched allogeneic HSCT and because of this have been investigated in order to understand their possible role in the control of GVHD and GvL. Diverse minor histocompatibility antigens of various genetic and cellular origins have been described. More than 40 different genes that encode mHAgs recognized by either CD8+ or CD4+ T cells have been identified. Most of the mHAgs are the result of non-synonymous single nucleotide polymorphisms in autosomal genes while others are encoded by the sex chromosomes. At least 6 genes in the Y chromosome encode male-specific MHAgs (so-called HY antigens). Additionally, mHAgs may also be caused by gene deletions and genetic variations in non-coding regions affecting gene transcription. The best-characterized minor histocompatibility antigen is encoded by the Y chromosome (HA-1). The mHAgs related to gender seem to be involved in HSCT outcomes because their absence in women can lead to a response to male antigens; female-to-male transplants seem to be more susceptible to GVHD. Antibody responses to HY proteins are also associated with both chronic GVHD and the maintenance of remission, but whether these antibody responses contribute meaningfully to GVHD, or simply serve as markers for it, remains unclear. In spite of female-to-male immune responses being more common, the opposite can also happen. Some mHAgs are expressed only in the hematopoietic system while others are also expressed in normal tissues. mHAgs whose expression is limited to hematopoietic tissue may be recognized by specific donor T cells and may selectively contribute to a GvL effect and those with broad tissue expression may mediate GVHD.
References
- Weatherbee BAT, Cui T, Zernicka-Goetz M. (2021). Modeling human embryo development with embryonic and extra-embryonic stem cells,Dev Biol.
View at Publisher | View at Google Scholar - Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. (2019). Stem cells: past, present, and future. Stem Cell Research & Therapy, 10(1):68.
View at Publisher | View at Google Scholar - Pellin D, Loperfido M, Baricordi C, Wolock SL, Montepeloso A, Weinberg OK, Biffi A, Klein AM, Biasco L. (2019). A comprehensive single cell transcriptional landscape of human hematopoietic progenitors, Nat Commun, 3:10(1):23-95.
View at Publisher | View at Google Scholar - Gschwandtner M, Kienzl P, Tajpara P, Schuster C, Stipek G, Buchberger M, Mildner M, Mairhofer M, Eppel W, Vierhapper M, Pammer J, Koller R, Elbe-Bürger A, Tschachler E. (2018). The Reticulum-Associated Protein RTN1A Specifically Identifies Human Dendritic Cells,J Invest Dermatol, 138(6):1318-1327.
View at Publisher | View at Google Scholar - Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, Chadburn A, Murphy AJ, Valenzuela DM, Gale NW, Thurston G, Yancopoulos GD, D'Angelica M, Kemeny N, Lyden D, Rafii S. (2008). CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors,J Clin Invest, 118(6):2111-2120.
View at Publisher | View at Google Scholar - Kukal S, Guin D, Rawat C, Bora S, Mishra MK, Sharma P, Paul PR, Kanojia N, Grewal GK, Kukreti S, Saso L, Kukreti R. (2021). Multidrug efflux transporter ABCG2: expression and regulation. Cell Mol Life Sci, 78(21-22):6887-6939.
View at Publisher | View at Google Scholar - Morcos MNF, Schoedel KB, Hoppe A, Behrendt R, Basak O, Clevers HC, Roers A, Gerbaulet A. (2017). SCA-1 Expression Level Identifies Quiescent Hematopoietic Stem and Progenitor Cells. Stem Cell Reports, 6:8(6):1472-1478.
View at Publisher | View at Google Scholar - Pedica F, Beccari S, Pedron S, Montagna L, Piccoli P, Doglioni C, Chilosi M. (2014). PDX-1 (pancreatic/duodenal homeobox-1 protein 1). Pathologica, 106(4):315-321.
View at Publisher | View at Google Scholar - Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. (2014). Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 32(6):1380-1389.
View at Publisher | View at Google Scholar - La Rocca G, King B, Shui B, Li X, Zhang M, Akat KM, Ogrodowski P, Mastroleo C, Chen K, Cavalieri V, Ma Y, Anelli V, Betel D, Vidigal J, Tuschl T, Meister G, Thompson CB, Lindsten T, Haigis K, Ventura A. (2021). Inducible and reversible inhibition of miRNA-mediated gene repression in vivo. Elife, 31:10:709-748.
View at Publisher | View at Google Scholar - Iancu-Rubin C, Hoffman R. (2015). Role of epigenetic reprogramming in hematopoietic stem cell function. CurrOpinHematol, 22(4):279-285.
View at Publisher | View at Google Scholar - Lee J, Go Y, Kang I, Han YM, Kim J. (2010). Oct-4 controls cell-cycle progression of embryonic stem cells. Biochem J, 9:426(2):171-181.
View at Publisher | View at Google Scholar - Whetton AD, Spooncer E. (1998). Role of cytokines and extracellular matrix in the regulation of haemopoietic stem cells. CurrOpin Cell Biol,10(6):721-726.
View at Publisher | View at Google Scholar - Furukawa Y. (2002). Cell cycle control genes and hematopoietic cell differentiation. Leuk Lymphoma, 43(2):225-231.
View at Publisher | View at Google Scholar - Maneix L, Iakova P, Moree SE, Hsu JI, Mistry RM, Stossi F, Lulla P, Sun Z, Sahin E, Yellapragada SV, Catic A. (2022). Proteasome Inhibitors Silence Oncogenes in Multiple Myeloma through Localized Histone Deacetylase 3 (HDAC3) Stabilization and Chromatin Condensation. Cancer Res Commun, 2(12):1693-1710.
View at Publisher | View at Google Scholar - Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl AcadSci U S A, 5:97(25):13625-13630.
View at Publisher | View at Google Scholar - Kuroda Y, Dezawa M. (2014). Mesenchymal stem cells and their subpopulation, pluripotent muse cells, in basic research and regenerative medicine. Anat Rec (Hoboken), 297(1):98-110.
View at Publisher | View at Google Scholar - Brukman NG, Uygur B, Podbilewicz B, Chernomordik LV. (2019). How cells fuse. J Cell Biol,218(5):1436-1451.
View at Publisher | View at Google Scholar - Fu, S., and J. Liesveld. (2000).
View at Publisher | View at Google Scholar - Rettig, Michael P, George Ansstas, and John F. DiPersio. (2012). “Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4.” Leukemia ,26.1:34-53.
View at Publisher | View at Google Scholar - Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R, Guetta E, Barkai G, Nagler A, Lapidot T. (2001). Rapid and efficient homing of human CD34(+) CD38(-/low) CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood,15;97(10):3283-3291.
View at Publisher | View at Google Scholar - Williams, K., Motiani, K., Giridhar, P. V., & Kasper, S. (2013). CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Experimental Biology and Medicine.
View at Publisher | View at Google Scholar - Papayannopoulou T. (2000). Mechanisms of stem-/progenitor-cell mobilization: the anti-VLA-4 paradigm. Semimetal, (1Suppl2):11-8.
View at Publisher | View at Google Scholar - Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. (1998). Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. :393(6685):595-599.
View at Publisher | View at Google Scholar - Mendt M, Cardier JE. (2012). Stromal-derived factor-1 and its receptor, CXCR4, are constitutively expressed by mouse liver sinusoidal endothelial cells: implications for the regulation of hematopoietic cell migration to the liver during extramedullary hematopoiesis. Stem Cells Dev, 21(12):2142-2151.
View at Publisher | View at Google Scholar - Mitroulis I, Kalafati L, Hajishengallis G, Chavakis T. (2018). Myelopoiesis in the Context of Innate Immunity. J Innate Immun, (5-6):365-372.
View at Publisher | View at Google Scholar - Ando K. (2002). Human CD34- hematopoietic stem cells: basic features and clinical relevance. Int J Hematol,75(4):370-375.
View at Publisher | View at Google Scholar - Uchida N, Li L, Nassehi T, Drysdale CM, Yapundich M, Gamer J, Haro-Mora JJ, Demirci S, Leonard A, Bonifacino AC, Krouse AE, Linde NS, Allen C, Peshwa MV, De Ravin SS, Donahue RE, Malech HL, Tisdale JF. (2021). Preclinical evaluation for engraftment of CD34+ cells gene-edited at the sickle cell disease locus in xenograft mouse and non-human primate models. Cell Rep Med, 2(4):100-247.
View at Publisher | View at Google Scholar - Baumeister SHC, Rambaldi B, Shapiro RM, Romee R. (2020). Key Aspects of the Immunobiology of Haplo-identical Hematopoietic Cell Transplantation. Front Immunol,11:191.
View at Publisher | View at Google Scholar - Yamanaka S. (2020). Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell, 27(4):523-531.
View at Publisher | View at Google Scholar - Caldwell KL, Wang J. (2015). Cell-based articular cartilage repair: the link between development and regeneration. Osteoarthritis Cartilage, 23(3):351-362.
View at Publisher | View at Google Scholar - Laskowski TJ, Biederstädt A, Rezvani K. (2002). Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer, 22(10):557-575.
View at Publisher | View at Google Scholar - Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. (2002). Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science,295(5562):2097-2100.
View at Publisher | View at Google Scholar - Gao F, Ye Y, Gao Y, Huang H, Zhao Y. (2020). Influence of KIR and NK Cell Reconstitution in the Outcomes of Hematopoietic Stem Cell Transplantation. Front Immunol,11:20-22.
View at Publisher | View at Google Scholar - Sivori S, Pende D, Quatrini L, Pietra G, Della Chiesa M, Vacca P, Tumino N, Moretta F, Mingari MC, Locatelli F, Moretta L. (2021). NK cells and ILCs in tumor immunotherapy. Mol Aspects Med,80:100-870.
View at Publisher | View at Google Scholar - Dhuyser A, Aarnink A, Pérès M, Jayaraman J, Nemat-Gorgani N, Rubio MT, Trowsdale J, Traherne J. (2022). KIR in Allogeneic Hematopoietic Stem Cell Transplantation: Need for a Unified Paradigm for Donor Selection. Front Immunol,13:821-533.
View at Publisher | View at Google Scholar - Weisdorf D, Cooley S, Wang T, Trachtenberg E, Vierra-Green C, Spellman S, Sees JA, Spahn A, Vogel J, Fehniger TA, Woolfrey AE, Devine SM, Ross M, Waller EK, Sobecks RM, McGuirk J, Oran B, Farag SS, Shore T, Van Besien K, Marsh SGE, Guethlein LA, Parham P, Miller JS. (2020). KIR B donors improve the outcome for AML patients given reduced intensity conditioning and unrelated donor transplantation. Blood Adv. 4(4):740-754.
View at Publisher | View at Google Scholar - Martín-Antonio B, Granell M, Urbano-Ispizua A. Genomic polymorphisms of the innate immune system and allogeneic stem cell transplantation. Expert Rev Hematol. 2010 Aug;3(4):411-27. doi: 10.1586/ehm.10.40. PMID: 21083033.
View at Publisher | View at Google Scholar - Machuldova A, Houdova L, Kratochvilova K, Leba M, Jindra P, Ostasov P, Maceckova D, Klieber R, Gmucova H, Sramek J, Holubova M. (2021). Single-Nucleotide Polymorphisms in MICA and MICB Genes Could Play a Role in the Outcome in AML Patients after HSCT. J Clin Med,10(20):46-36.
View at Publisher | View at Google Scholar - Fuertes MB, Domaica CI, Zwirner NW. (2021). Leveraging NKG2D Ligands in Immuno-Oncology. Front Immunol,12:7131-7158.
View at Publisher | View at Google Scholar - Boukouaci W, Busson M, Peffault de Latour R, Rocha V, Suberbielle C, Bengoufa D, Dulphy N, Haas P, Scieux C, Amroun H, Gluckman E, Krishnamoorthy R, Toubert A, Charron D, Socié G, Tamouza R. (2009). MICA-129 genotype, soluble MICA, and anti-MICA antibodies as biomarkers of chronic graft-versus-host disease. Blood, 114(25):5216-5224.
View at Publisher | View at Google Scholar - hoi EY, Choi K, Nam G, Kim W, Chung M. (2020). H60: A Unique Murine Hematopoietic Cell-Restricted Minor Histocompatibility Antigen for Graft-versus-Leukemia Effect. Front Immunol, 11:11-63.
View at Publisher | View at Google Scholar

 Clinic
Clinic
