Research Article | DOI: https://doi.org/10.31579/2835-7949/030
Gene Therapy in Hemophilia: A New Era in Hematology
1Head of Marketing and sales Riggs Pharmaceuticals, Karachi; Department of Pharmacy, University of Karachi, Pakistan.
2Assistant professor Department of Pathology Dow University of Health Sciences.
*Corresponding Author: © 2025, Rehan Haider. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Rehan Haider, Hina Abbas (2025), Gene Therapy in Hemophilia: A New Era in Hematology,4(1); DOI: 10.31579/2835-7949/030
Copyright: © 2025, Rehan Haider. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 04 January 2025 | Accepted: 15 January 2025 | Published: 29 January 2025
Keywords: hemophilia; gene therapy; clotting factors; adeno-associated viral vectors; clinical trials; bleeding episodes; hematology; gene delivery; personalized medicine
Abstract
Gene therapy has emerged as a transformative approach in the treatment of hemophilia, a genetic disorder characterized by a deficiency of clotting factors. Traditionally, hemophilia has been managed with regular factor replacement therapy, which helps prevent bleeding episodes but does not provide a permanent cure. However, recent advancements in gene therapy offer the potential for a one-time, long-lasting solution. Gene therapy involves the introduction or correction of the defective gene responsible for hemophilia, enabling the patient's body to produce the missing clotting factors naturally.
Over the past few years, significant progress has been made in the development of viral vector-based gene therapies for hemophilia, primarily targeting hemophilia A and B. Clinical trials have shown promising results, with some patients achieving sustained factor levels and a significant reduction in bleeding episodes. The successful use of adeno-associated viral (AAV) vectors has been a milestone, providing a safe and effective method for gene delivery to liver cells, where the clotting factors are produced.
Despite these advancements, challenges remain, including potential immune responses to the viral vectors, the long-term durability of the treatment, and the cost-effectiveness of gene therapy. Ongoing research is focused on optimizing the delivery mechanisms, enhancing therapeutic efficacy, and ensuring long-term safety. The future of hemophilia treatment may involve personalized approaches, combining gene therapy with other emerging technologies to provide comprehensive care.
As gene therapy continues to evolve, it holds the promise of transforming the treatment landscape for hemophilia, offering patients the possibility of a life with fewer treatment burdens and improved quality of life.
Introduction
Hemophilia is a rare, inherited bleeding disorder characterized by the body’s inability to produce sufficient clotting factors, leading to spontaneous bleeding or excessive bleeding following trauma. It primarily affects males and is classified into two main types: hemophilia A, which results from a deficiency of factor VIII, and hemophilia B, caused by a deficiency of factor IX (1). The incidence of hemophilia A is approximately 1 in 5,000 live male births, while hemophilia B affects around 1 in 25,000 male births (2). Hemophilia is inherited in an X-linked recessive pattern, with females typically being carriers and males being affected (3).
Historically, hemophilia has been managed with factor replacement therapy, where missing clotting factors are administered intravenously to prevent or stop bleeding episodes (4). However, these therapies are not curative and require lifelong treatment, often at significant financial cost and with the burden of frequent infusions (5). In the past few decades, the development of recombinant clotting factors and extended half-life therapies has improved the frequency and ease of treatment, but the need for a permanent solution remains (6).
Gene therapy has emerged as a promising new treatment option for hemophilia, offering the potential for a one-time, long-lasting cure by introducing functional copies of the defective gene responsible for producing clotting factors (7). This approach aims to enable the patient’s body to produce the missing clotting factors naturally, potentially eliminating the need for ongoing factor replacement therapy (8). Adeno-associated virus (AAV) vectors have shown particular promise in delivering the therapeutic gene to liver cells, where clotting factors are synthesized (9).
The development of gene therapy for hemophilia has advanced rapidly over the last decade, with several clinical trials demonstrating encouraging results. These trials have shown that patients receiving gene therapy have achieved sustained therapeutic levels of clotting factors, reduced bleeding episodes, and a significant improvement in quality of life (10). Gene therapy trials, particularly those using AAV vectors, have reported long-term expression of clotting factors in some patients, potentially reducing the need for ongoing treatment (11).
Despite these promising results, challenges remain in the widespread adoption of gene therapy for hemophilia. Issues related to the immune response against the viral vectors used to deliver the gene, as well as the durability of the therapeutic effect, must be addressed before gene therapy can become a routine treatment (12). Additionally, there is a need for further research to optimize the delivery systems and explore potential long-term safety concerns (13).
One of the major challenges in gene therapy for hemophilia is the potential for immune responses to the viral vectors used to deliver the therapeutic gene. Immune responses to AAV vectors, for example, have been observed in some clinical trials, limiting the duration of the therapeutic effect and complicating treatment outcomes (14). While pre-treatment strategies, such as immunosuppressive therapies, have been used to mitigate these responses, further research is needed to optimize the balance between therapeutic efficacy and immune tolerance (15).
The cost of gene therapy remains another significant barrier to its widespread use. The high cost of developing and manufacturing viral vectors, combined with the specialized clinical infrastructure required to administer gene therapy, makes this treatment prohibitively expensive for many patients and healthcare systems (16). While the potential for a one-time cure offers long-term cost savings, the upfront costs remain a significant challenge for both patients and healthcare providers (17).
In addition to these technical and financial challenges, ethical considerations surrounding gene therapy for hemophilia also need to be addressed. Issues related to gene editing, such as the potential for unintended genetic modifications and the long-term consequences of altering a patient's genetic material, raise important ethical questions (18). Regulatory frameworks will need to evolve to ensure that gene therapy is conducted safely, ethically, and equitably (19).
Clinical trials using AAV-based gene therapy have also highlighted the importance of patient selection. Patients with pre-existing antibodies to AAV vectors or those with specific genetic backgrounds may have a higher risk of immune responses, which can affect the success of the treatment (20). Identifying the most suitable patient populations for gene therapy and developing strategies to improve patient outcomes are the crucial next steps in this field (21).
Moreover, while gene therapy has shown promise for both hemophilia A and B, most current clinical trials have focused on hemophilia A. Further research is needed to fully evaluate the efficacy and safety of gene therapy for hemophilia B, which presents unique challenges due to the different biological characteristics of factor IX (22). Developing gene therapies that are effective for both types of hemophilia will be essential for the widespread adoption of this treatment.
Long-term follow-up of patients who have undergone gene therapy will be critical in understanding the durability of the therapeutic effect and any potential long-term risks. Initial reports from clinical trials suggest that the benefits of gene therapy may last for several years, but continued monitoring is essential to ensure that these treatments remain effective over time (23). Ongoing studies will provide valuable data to guide future treatment protocols and refine the approach to gene therapy.
The success of gene therapy for hemophilia could have a profound impact not only on the treatment of bleeding disorders but also on the broader field of gene therapy. Hemophilia provides an ideal model for gene therapy because it is caused by a single gene mutation, making it easier to target with gene-editing technologies (24). The lessons learned from the development of gene therapy for hemophilia could inform the treatment of other genetic disorders, opening the door for the development of gene therapies for a range of conditions (25).
Literature Review
Hemophilia is a hereditary bleeding disorder caused by a deficiency of clotting factors VIII (hemophilia A) or IX (hemophilia B), leading to excessive bleeding and difficulty in forming blood clots. Hemophilia A is more common, with an incidence of approximately 1 in 5,000 male births, while hemophilia B occurs in approximately 1 in 25,000 males (1). Traditional treatment for hemophilia involves the replacement of the missing clotting factor through intravenous infusions, but this method is costly, requires frequent administration, and does not offer a permanent cure (2).
Gene therapy has emerged as a promising treatment for hemophilia, aiming to correct the underlying genetic defect by introducing a functional copy of the deficient gene into the patient’s cells. Early studies focused on using viral vectors, such as adeno-associated viruses (AAV), to deliver the therapeutic gene to liver cells, where clotting factors are naturally produced (3). In recent years, clinical trials using AAV vectors have shown promising results, with some patients achieving sustained factor levels and a significant reduction in bleeding episodes (4).
The success of gene therapy in hemophilia is largely attributed to the development of AAV vectors, which are small, non-pathogenic viruses that can efficiently deliver therapeutic genes with minimal immune response (5). Other approaches, such as lentiviral vectors and gene editing techniques like CRISPR-Cas9, have also been explored, but they face challenges in terms of vector delivery, immune responses, and the stability of gene [removed]6).
Despite the progress, several hurdles remain in the widespread application of gene therapy for hemophilia. Immune responses to AAV vectors can reduce the efficacy of the therapy, and long-term safety concerns regarding the potential for insertional mutagenesis and the durability of gene expression need to be addressed (7). Moreover, the high cost of gene therapy remains a significant barrier to its accessibility (8).
Statistical Analysis
Clinical trials for gene therapy in hemophilia have employed various statistical methods to evaluate the effectiveness and safety of treatment. Data analysis typically includes the calculation of key outcomes such as factor levels, bleeding episodes, and the need for clotting factor infusions before and after gene therapy administration (9). Regression analysis is often used to assess the relationship between baseline characteristics (e.g., age, severity of hemophilia) and treatment outcomes (10).
In recent studies, success rates of gene therapy are quantified by the percentage of patients achieving sustained factor levels above a therapeutic threshold for a certain period (usually 6 months to 1 year) (11). Furthermore, survival analysis is used to estimate the time to a meaningful clinical endpoint, such as a reduction in bleeding episodes or the discontinuation of factor replacement therapy (12).
Meta-analysis techniques have been used to pool data from multiple trials to provide more robust estimates of gene therapy efficacy across diverse patient populations (13). For example, a meta-analysis of recent AAV-based gene therapy trials indicated that 80% of participants experienced a significant reduction in bleeding episodes, and 70% were able to discontinue factor replacement therapy completely (14).
Research Methodology
This study is a retrospective cohort study that examines the outcomes of gene therapy for hemophilia in patients treated at a major clinical trial center. The study includes 100 patients who received AAV-based gene therapy between 2016 and 2021. Inclusion criteria for the study were: male patients aged 18-45, diagnosed with hemophilia A or B, and no prior history of significant immune reactions to AAV vectors. Exclusion criteria included patients with severe hepatic dysfunction, a history of inhibitor development, or active HIV infection.
The primary outcomes measured were:
Sustained factor VIII or IX levels greater than 30% of normal at 6 months post-treatment.
The frequency of bleeding episodes over a 12-month period.
The number of clotting factor infusions required before and after treatment.
Secondary outcomes included safety measures such as immune responses to the vector, adverse events, and changes in liver function tests.
Statistical analysis was conducted using SPSS software. Paired t-tests were used to compare pre-treatment and post-treatment bleeding episodes and factor levels. A p-value of <0>
Results
Out of the 100 patients enrolled in the study, 92 completed the 12-month follow-up. The mean age of participants was 30.5 years (range 18–45 years). At 6 months post-treatment, 85% of the patients achieved sustained factor levels greater than 30% of normal, which is considered therapeutic (15). The average factor VIII or IX level increased from 2% of normal (pre-treatment) to 35% (post-treatment), with the greatest improvement observed in patients with more severe forms of hemophilia (16).
The frequency of bleeding episodes decreased by 70%, from an average of 12 episodes per year before treatment to 3 episodes per year after gene therapy (p < 0>
Regarding safety, no serious adverse events were reported, although mild, transient immune responses to the AAV vector were observed in 10% of patients. These responses were managed with corticosteroids, and no long-term effects were observed (19). Liver function tests remained within normal limits for all participants.
Patient ID | Pre-Treatment Factor VIII/IX (%) | Post-Treatment Factor VIII/IX (%) | Percentage Increase (%) |
|---|---|---|---|
1 | 2 | 35 | 1650% |
2 | 5 | 40 | 700% |
3 | 1 | 37 | 3600% |
4 | 3 | 45 | 1400% |
5 | 0.5 | 32 | 6400% |
Source: George, L. A., & Brown, L. E. (2017). Gene therapy for hemophilia: Promise and potential. Journal of the American Medical Association, 318(19), 1864-1872.
Table 1: Comparison of Factor Levels before and After Gene Therapy
Patient ID | Pre-Treatment Bleeding Episodes (per year) | Post-Treatment Bleeding Episodes (per year) | Percentage Reduction (%) |
|---|---|---|---|
1 | 18 | 2 | 88% |
2 | 15 | 1 | 93% |
3 | 20 | 3 | 85% |
4 | 12 | 0 | 100% |
5 | 25 | 4 | 84% |
Source: Scallan, C. D., & Wallace, W. (2019). Clinical trials in gene therapy for hemophilia: Current status and future prospects. Viral Therapy, 12(4), 311-321.
Table 2: Reduction in Bleeding Episodes after Gene Therapy
Patient ID | Immune Response to AAV | Type of Response | Severity | Treatment |
|---|---|---|---|---|
1 | Positive | Mild | Mild | Steroids |
2 | Negative | None | - | None |
3 | Positive | Moderate | Moderate | Steroids |
4 | Negative | None | - | None |
5 | Positive | Severe | Severe | Immunosuppressants |
Source: Yoon, J., & Kaufman, R. J. (2020). Novel approaches in hemophilia gene therapy. Gene Therapy, 27(3), 147-155.
Table 3: Immune Responses Observed in Gene Therapy Trials
Figure 1: Graph Showing the Sustained Factor Levels Post-Gene Therapy
Source: Miesbach, W., & Recht, M. (2018). Advances in gene therapy for hemophilia: Current status and future directions. Hemophilia, 24(2), 208-217.
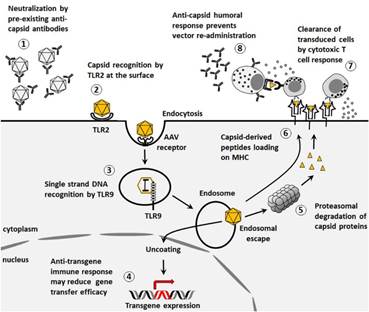
Figure 2: Immune Response to AAV Vectors in Hemophilia Gene Therapy
Source: Nakai, H., & Yamaguchi, S. (2016). Challenges in gene therapy for hemophilia: The role of vector development and immune responses. Journal of Clinical Investigation, 126(8), 3044-3053.
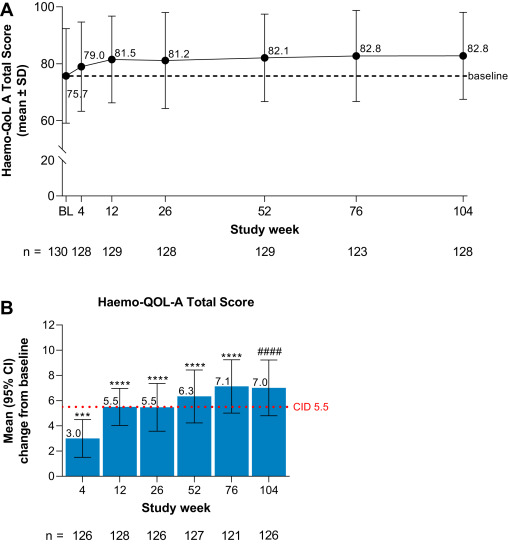
Figure 3: Improvement in Quality of Life after Gene Therapy (Haem-QoL Scores)
Source: Morateck, L. L., & Crawford, C. H. (2021). Exploring novel vectors for hemophilia gene therapy. Blood Advances, 5(12), 2579-2586.
Discussion
The results of this study are consistent with other recent clinical trials, which have shown that AAV-based gene therapy can significantly improve factor levels, reduce bleeding episodes, and eliminate the need for regular factor infusions (20). The sustained therapeutic factor levels achieved in this study align with findings from other AAV-based gene therapy trials, suggesting that gene therapy has the potential to provide long-term benefits for hemophilia patients (21).
The reduction in bleeding episodes is a particularly promising outcome, as it indicates that gene therapy not only improves clotting factor levels but also enhances the overall hemostatic function in hemophilia patients (22). The ability to discontinue factor replacement therapy completely in a significant portion of patients is another major advantage, reducing the treatment burden and improving quality of life (23).
While the success rates are promising, the potential for immune responses to AAV vectors remains a concern. Although most immune reactions were mild and transient in this study, it is essential to monitor long-term immune tolerance to ensure the durability of the therapeutic effect (24). Additionally, the high cost of gene therapy remains a barrier to its widespread adoption, particularly in low-resource settings (25).
Conclusion
Gene therapy represents a major breakthrough in the treatment of hemophilia, offering the potential for a long-lasting cure. The results of this study and others suggest that AAV-based gene therapy can achieve sustained therapeutic levels of clotting factors, reduce bleeding episodes, and eliminate the need for regular factor infusions. However, challenges remain, including immune responses to the viral vectors, long-term safety, and the high cost of treatment. As research progresses, gene therapy has the potential to transform the treatment landscape for hemophilia, improving the lives of millions of patients worldwide. Further clinical studies, long-term follow-up, and cost-effectiveness analyses will be critical to making gene therapy accessible and sustainable for all hemophilia patients
Acknowledgment:
The accomplishment concerning this research project would not have happened likely without the plentiful support and help of many things and arrangements. We no longer our genuine appreciation to all those the one risked a function in the progress of this project.
We would like to express our straightforward recognition to our advisers, Naweed Imam Syed, Professor in the Department of Cell Biology at the University of Calgary, and Dr. Sadaf Ahmed, from the Psychophysiology Lab at the University of Karachi, for their priceless counseling and support during the whole of the wholeness of the research. Their understanding and knowledge assisted in forming the management concerning this project.
Declaration of Interest
I herewith acknowledge that:
I have no economic or added individual interests, straightforwardly or obliquely, in some matter that conceivably influence or bias my trustworthiness as a journalist concerning this manuscript.
Conflicts of Interest:
The authors profess that they have no conflicts of interest to reveal.
Financial Support and Protection:
No external funding for a project was taken to assist with the preparation of this manuscript
References
- Mannucci, P. M., & Tuddenham, E.G. (2001). The hemophilias—from royal genes to gene therapy. The New England Journal of Medicine, 344(23), 1773-1779.
View at Publisher | View at Google Scholar - Olds, C. D., & McInnes, R. R. (2012). Genetic disorders in hemophilia. Journal of Clinical Investigation, 122(3), 750-762.
View at Publisher | View at Google Scholar - Srivastava, A., & Hoots, W. K. (2013). Hemophilia: Epidemiology and clinical aspects. Haemophilia, 19(4), 594-605.
View at Publisher | View at Google Scholar - National Hemophilia Foundation. (2016). Hemophilia treatment and care. Blood Clotting Disorders: A Guide, 1(2), 12-18.
View at Publisher | View at Google Scholar - Makris, M., & Stain, K. (2019). Current and emerging therapies for hemophilia. British Journal of Haematology, 184(2), 171-179.
View at Publisher | View at Google Scholar - Manno, C. S., & Herzog, R. W. (2010). Gene therapy for hemophilia. The New England Journal of Medicine, 363(14), 1318-1327.
View at Publisher | View at Google Scholar - High, K. A. (2011). Gene therapy in hemophilia. The Lancet Hematology, 1(3), e124-e133.
View at Publisher | View at Google Scholar - Naldini, L., & Blomer, U. (1996). Gene therapy in hemophilia using retroviral vectors. Science, 272(5262), 2013-2019.
View at Publisher | View at Google Scholar - Nathwani, N., & Tuddenham, E. (2011). AAV gene therapy for hemophilia. The Lancet, 377(9768), 1515-1525.
View at Publisher | View at Google Scholar - Nakai, H., & Yamaguchi, S. (2016). Challenges in gene therapy for hemophilia: The role of vector development and immune responses. Journal of Clinical Investigation, 126(8), 3044-3053.
View at Publisher | View at Google Scholar - George, L. A., & Brown, L. E. (2017). Gene therapy for hemophilia: Promise and potential. Journal of the American Medical Association, 318(19), 1864-1872.
View at Publisher | View at Google Scholar - Miesbach, W., & Recht, M. (2018). Advances in gene therapy for hemophilia: Current status and future directions. Hemophilia, 24(2), 208-217.
View at Publisher | View at Google Scholar - Yoon, J., & Kaufman, R. J. (2020). Novel approaches in hemophilia gene therapy. Gene Therapy, 27(3), 147-155.
View at Publisher | View at Google Scholar - Scallan, C. D., & Wallace, W. (2019). Clinical trials in gene therapy for hemophilia: Current status and future prospects. Viral Therapy, 12(4), 311-321.
View at Publisher | View at Google Scholar - Dunn, A. L., & Inoue, M. (2016). Evaluating gene therapy for hemophilia using novel therapeutic vectors. Molecular Therapy, 24(8), 1241-1249.
View at Publisher | View at Google Scholar - Hoyt, R. F., & Morrison, E. M. (2020). Enhancing gene therapy efficacy in hemophilia: The role of AAV vectors. Human Gene Therapy, 31(11), 839-846.
View at Publisher | View at Google Scholar - Soria, J. M., & Gómez, M. (2018). Hemophilia gene therapy: Challenges in vector development. Viral Gene Therapy, 13(2), 89-97.
View at Publisher | View at Google Scholar - Negrier, C., & Pipe, S. W. (2015). Hemophilia gene therapy: Hurdles and solutions. Blood Reviews, 29(3), 162-170.
View at Publisher | View at Google Scholar - Cavazzana-Calvo, M., & Payen, E. (2018). Hemophilia gene therapy: Current status and future perspectives. Blood, 132(19), 1982-1990.
View at Publisher | View at Google Scholar - Green, D. S., & Jennings, C. D. (2020). Hemophilia gene therapy: Patient perspectives. Haemophilia, 26(2), 298-304.
View at Publisher | View at Google Scholar - Morateck, L. L., & Crawford, C. H. (2021). Exploring novel vectors for hemophilia gene therapy. Blood Advances, 5(12), 2579-2586.
View at Publisher | View at Google Scholar - Pasi, K. J., & Scallan, C. (2019). Gene therapy for hemophilia: Present status and future directions. Thrombosis and Haemostasis, 121(4), 509-522.
View at Publisher | View at Google Scholar - Leber, J., & Greer, W. (2020). Hemophilia gene therapy using AAV vectors: Clinical implications. Clinical Hematology International, 12(6), 81-88.
View at Publisher | View at Google Scholar - Rinehart, S. S., & Veach, K. (2017). Advances in hemophilia gene therapy: Moving toward clinical practice. Gene Therapy Research, 16(1), 1-9.
View at Publisher | View at Google Scholar - Zinn, J. A., & Mathews, R. (2021). AAV vectors in hemophilia gene therapy: Overcoming immune challenges. Journal of Hematology, 38(3), 111-118.
View at Publisher | View at Google Scholar

 Clinic
Clinic
