Research Article | DOI: https://doi.org/10.31579/2834-8761/003
Evaluation Of In Vitro And In Vivo Antioxidant Activities of Methanolic Leaf Extract of Anthocleista Djalonensis in Triton Wr-1339 Induced Toxicity
- Adebayo A. OGUNBOYE 1*
- Mary T. OLALEYE 1
- Afolabi C. AKINMOLADUN 1
- Olamide O. CROWN 2
- Olanrewaju Sam Olayeriju 3
1 Phytomedicine, Biochemical and Molecular Pharmacology and Toxicology Laboratories Department of Biochemistry, School of Life Sciences, Federal University of Technology, P. M. B. 704, Akure, Nigeria
2 Department of Chemistry, Physics and Atmospheric Sciences. Jackson States University, Jackson, Mississippi, USA.
3 Department of chemical sciences (Biochemistry), School Sciences, Olusegun Agagu University of Technology, PMB 353, Okitipupa, Nigeria
*Corresponding Author: Adebayo A. Phytomedicine, Biochemical and Molecular Pharmacology and Toxicology Laboratories Department of Biochemistry, School of Life Sciences, Federal University of Technology, P. M. B. 704, Akure, Nigeria
Citation: Adebayo A. OGUNBOYE, Mary T. OLALEYE, Afolabi C. AKINMOLADUN, Olamide O. CROWN, et al. (2022). Evaluation Of In Vitro And In Vivo Antioxidant Activities of Methanolic Leaf Extract of Anthocleista Djalonensis in Triton Wr-1339 Induced Toxicity, J. Clinical Endocrinology and Metabolism, 1(1) DOI: 10.31579/2834-8761/003
Copyright: © 2022 Adebayo A. OGUNBOYE. This is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Received: 06 September 2022 | Accepted: 23 September 2022 | Published: 03 October 2022
Keywords: (ADL: Anthocleista djalonensis leaf; SOD: superoxide dismutase; GPx: glutathione peroxidase; CAT: catalase; GSH: reduced glutathione; FRAP: ferric reducing antioxidant power.
Abstract
Anthocleista djalonensis A. Chev (Gentianaceae), a wild tropical perennial herb called Cabbage tree is used by local practitioners for the several medicinal purposes. The present study evaluated the in vitro antioxidant activities, in vivo antioxidant activities in triton-induced toxified rats and the GC-MS analysis of the methanolic leaf extract. The phytochemical investigation revealed the presence of tannins, flavonoids, saponins, cardiac glycosides, anthraquinones, terpenoids and steroids. Quantitative antioxidant evaluation of ADL gave total tannin, total phenolic content, total flavonoid content, and total antioxidant capacity as 192.24±6.06 mg/g catechin equivalent, 78.71±3.03 mg/g gallic acid equivalent, 154.67±1.77 mg/g quercetin equivalent, and 112.33±4.50 mg/g ascorbic acid equivalent. ADL demonstrated appreciable in vitro antioxidant capacity and radical scavenging ability compared with reference standards. Toxicity was induced in wistar rats by single intraperitoneal (i.p) injections of Triton X-1339 at a dose of 200 mg/kg b.w. Twenty four hours after Triton induction different dosages of ADL (200 mg/kg b.w., 400 mg/kg b.w., 600 mg/kg b.w.). Plant extract was administered for 14 consecutive days. Animals were sacrificed 24 hours after the last administration. Heart and liver were collected for biochemical analysis. ADL restored cardiac and hepatic antioxidant status by significantly lower mean levels of GPx, SOD, GSH, CAT and FRAP. ADL also showed significant protection against triton induced lipid peroxidation; malondialdehyde (MDA) as a biomarker of oxidative stress. These findings suggest that the leaf of Anthocleista djalonensis has potent antioxidant activity which may be responsible for some of its reported pharmacological activities and can be used as antioxidant supplement.
Introduction
Oxidative stress is the direct consequence of an increased generation of free radicals and or reduced physiological activity of antioxidant defenses against free radicals generated. (Khan et al., 2015). Free radicals are reactive chemical entities that are short lived species containing one or more unpaired electrons (Bansal and Bilaspuri, 2011). They can be classified into three types: reactive oxygen species (ROS) (e.g. superoxide - O-2, hydroxyl - OH‾, alkoxyl - RO‾, hydroperoxyl - HO-2, hydrogen peroxide - H2O2), reactive nitrogen species (RNS) (e.g. nitric acid - NO-, nitrous acid – HNO2, peroxynitrite - ONOO-, peroxinitrous acid – ONOOH) (Droge, 2011), reactive chlorine species (RCS) (e.g. hypochlorus acid – HOCl) (Freidovich, 1999). They serve physiological functions including activation of different signaling pathways inside the cell, such as the mitogen activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) pathways that alter gene expression. Oxygen-derived free radicals and oxidants (reactive oxygen species, ROS) are formed continuously in small amounts during the normal metabolism of cells and are normally inactivated by endogenous scavenging mechanisms. ROS levels below the homeostatic set point may interrupt the physiological role of oxidants in cellular proliferation and host defense. Similarly, increased ROS may also be detrimental and lead to cell death or to acceleration in ageing and age-related diseases (Rahman et al., 2012). Traditionally, the impairment caused by increased ROS is thought to result from random damage to proteins, lipids and DNA. In addition to these effects, a rise in ROS levels may also constitute a stress signal that activates specific redox-sensitive signaling pathways. Once activated, these diverse signaling pathways may have either damaging or potentially protective functions (Finkel, 2000). Humans are constantly exposed to free radicals created by several factors (Uttara et al., 2009). The excessive free radical generation may induce a number of alterations of cell constituents, including inactivation of enzymes, generation of reactive nitrogen species, damage of nucleic acid bases and proteins, and peroxidation of membrane lipids (Ha et al., 2006).
Antioxidants are the chemicals or enzymes that react with the free radicals and protect the vital biomolecules from the damage by terminating the oxidative chain reaction. Antioxidants act as a defence mechanism that protect against deleterious effects of oxidative reaction produced by reactive oxygen species (ROS) in a biological system (Jayachitra and Krithiga, 2010). Reactive oxygen species not only are produced naturally in cell following stress or respiration but also have been reported to be produced by radiation, bacterial and viral toxin, smoking, alcohol, and psychological or emotional stress. Overproduction of ROS and/or inadequate antioxidants has been implicated in the pathogenesis and complications of some disease conditions like diabetes, Alzheimer’s disease, cancer, atherosclerosis, arthritis, neurodegenerative disease, and aging process (Khalaf et al., 2008; Patel et al., 2010). Antioxidants have been reported to prevent oxidative damage caused by ROS by reacting with free radicals, chelating, and catalytic metals and also by acting as oxygen scavengers (Shahidi and Wanasundara, 1992; B¨uy¨ukokuroˇglu, et al., 2001). There is growing evidence that antioxidants play a pivotal role in the prevention of heart disease, cancer, DNA degeneration, pulmonary disease, and neurological disorder (Percival, 1998). Recently, there has been an upsurge of interest in the therapeutic potential of plants as antioxidants in reducing oxidative tissue injuries (Patel et al., 2010). Plants, herbs, and spice, rich in phenolic compounds like flavonoids, have been demonstrated to have anti-inflammatory, antiallergenic, antiviral, antiaging, and anticarcinogenic activities which can be attributed to their antioxidant properties (Percival, 1998; Aqil et al., 2006).
Anthocleista djalonensis A. Chev belongs to the family Gentianaceae and the Yoruba people of South west Nigeria refers to it as “Ewe Shapo” (Onocha et al., 2003). The tree grows up to 15 m tall; bole up to 40 cm in diameter; twigs sometimes with 2 erect spines or small cushions above the leaf axils (Jensen and Schripsema, 2002). The Genus Anthocleista comprises 14 species (Neuwinger, 2000); the West Africa species have the same vernacular names (Cabbage tree) and are used by local practitioners for the same medicinal purpose. A. djalonensis is used in traditional medicine for different ailments; the anti-inflammatory activity of the leaves and roots has been reported (Baba and Usifoh, 2011, Okunrobo et al., 2009). The seeds, barks, leaves and roots are used as antipyretic, laxative, remedy for various stomach disorders, antithelmintic, antimycobacterial, anti-bacterial and wound healing (Okoli and Iroegbu, 2004; Chah et al., 2006; Nweze and Ngongeh, 2007; Esimone et al., 2009). The leaf, bark and root of A. djalonensis are used to treat hypertension. It is used as herbal remedy for epilepsy in Ghana (Oliver-Bever, 1986; Iwu, 2000). Burkill (1995) reported that the plant is used as febrifuge, abortifacient and pain killer. In African traditional medicine, the leaves, stems and roots of A. djalonensis is prepared as a decoction or macerated in water or alcohol, and the solution is given orally as a treatment for diabetes (Ampofo, 1977; Abuh et al., 1990; Madubunyi et al., 1994; Olowokudejo et al., 2008; Jiofack et al., 2010; Diallo et al., 2012; Soladoye et al., 2012; Tchacondo et al., 2012). The hypoglycemic effect of the leaves, stem bark and roots of A. djalonensis has been scientifically proven by in vitro and in vivo studies (Abuh et al., 1990; Olagunju et al., 1998; Mbouangouere et al., 2007; Okokon et al., 2012; Olubomehin et al., 2013; Osadebe et al., 2014a, 2014b; Sunday et al., 2014). Some of the other ethno-medical uses of the extract of Anthocleista djalonensis leaves, roots and stem bark include treatment of wound, constipation, diarrhoea, dysentery, abdominal pain (Okoli and Iroegbu, 2004), hepatitis, jaundice, cirrhosis, fungal skin infection, filarial worm infections, acute inflammation and boils on skin (Aiyeloja and Bellow, 2006), treatment of dropsy, swellings, oedema, liver problems, antidotes to venomous stings and bites and pain killer (Burkill, 1985). The present study aimed at establishing the in vitro and in vivo antioxidant potentials of the methanolic leaf extract of Anthocleista djalonensis
2.0 Materials and Methods
All chemicals were of analytical grade. Aluminum chloride (AlCl3), 5',5'- dithiobis-2-nitrobenzoic acid (DTNB), reduced glutathione (GSH), 2,4-dinitrophenol, adenosine triphosphate (ATP), Acetonitrile, formic acid, were purchased from Merck (Darmstadt, Germany). Quercetin, catechin, Hydrogen peroxide (H2O2), DPPH (2,2-diphenyl-1-picrylhydrazyl) radical, tannic acid, ascorbic acid, mannitol, folin-ciocalteau reagent, DMSO, thiobarbituric acid (TBA), 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were procured from Sigma Chemical Co. (St. Louis, MO, USA). Analytical grade-methanol (MeOH), Sodium acetate, magnesium chloride (MgCl2), trichloroacetic acid (TCA), ammonium molybdate, ferrous sulphate, potassium dichromate (K2Cr2O7), glacial acetic acid, ethylenediamine tetraacetic acid (EDTA), sodium chloride (NaCl) were obtained from BDH (Poole, U.K.) and Hopkins & Williams (U.K.). Sodium nitroprusside, Griess reagent: (1% (w/v) sulfanilamide, 2% (v/v) H3PO4 and 0.1% (w/v) naphthylethylene, potassium ferricyanide [K3Fe (CN) 6], 1, 10-phenanthroline, sodium dodecyl sulphate (SDS), ammonium molybdate. All other chemicals used in the experiment were of the highest grade commercially available.
2.2. Plant Material: Collection and Identification
Leaves of Anthocleista djalonensis were collected from Yasere village in Okitipupa, Nigeria. The plant was authenticated at the herbarium of Federal University of Technology Akure (FUTA) with a voucher number FUTA-0150.
2.3. Experimental animals
Healthy adult male wistar rats weighing 150 – 180 g were used for the study. The animals were housed at standard housing condition at the Department of Biochemistry animal house, the Federal University of Technology Akure, with temperature (27 ± 3 °C) under 12 h light: dark cycle and fed standard rodent chow (Vita feeds Nigeria limited) and water ad libitum. Experiments were performed in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines according to standard guidelines for laboratory animal care and use.
- Methods
2.4.1 Preparation of aqueous methanolic leave extract of Anthocleista djalonensis (ADL)
The air-dried leaves were subjected to a coarse powder using a dry grinder. The powdered leaves were soaked in 80% methanol for 72 hours and filtered using whatman filter paper no. 1 to obtain the aqueous methanolic extract (ADL). The filtered extracts were concentrated in a rotary evaporator and further concentrated to dryness using freeze dryer. The residue was kept at -20°C for further studies.
- Phytochemistry
Qualitative evaluation of phytochemicals including flavonoids, alkaloids, tannins, phlobatannins, glycosides steroid and terpenoids, was carried out using standard procedures (Sofowora, 1993).
- Evaluation of antioxidant components and activity
- Total Phenol Content Determination
The total phenolic content of extract was determined using the Folin-Ciocalteu’s method of Singleton et al., (1999) as modified by Hung et al., (2001). Exactly 0.1 ml of ADL (1mg/ml) was rapidly mixed with 0.1 ml of Folin Ciocalteu reagent, followed by the addition of 0.3 ml sodium carbonate (7.5%, w/v) solution. The mixture was incubated in the dark for 30 min. The absorbance of the blue colour was read at 760 nm after 30 min on a spectrophotometer. The total phenolic content was extrapolated from a standard curve using tannic acid or gallic acid (graded concentration, 50 – 250 µg/ml) as a standard. The amount of total phenolics was expressed as gallic acid equivalent (GAE, mg gallic acid/g sample) through the calibration curve of gallic acid.
- Estimation of the Concentration of Total Flavonoid
The total flavonoid concentration was determined spectrophotometrically based on the procedure of (Marinova et al., 2005). Briefly, 0.5 ml of ADL solution and standard (quercetin) at different concentrations (12.5 – 200 µg/ml) were taken in test tubes dissolved in methanol followed by the addition of 0.1 ml of 10% aluminum chloride solution. 0.1 ml of 1 M sodium acetate solution was added to the mixtures in the test tubes. Furthermore, each reaction test tube was then immediately diluted with 2.8 ml of distilled water and mixed to incubate for 30 min at room temperature to complete reaction. The absorbance of pink colored solution was noted at 415 nm using a spectrophotometer against blank methanol. Total Flavonoid Concentration (TFC) of the extract was expressed as quercetin equivalents (QE).
- Estimation of the Concentration of Total Tannins
The tannins were determined by Folin - Ciocalteu method as described by Broadhurst et al. (1978). About 0.1 ml ADLE was added to a volumetric flask (10 ml) containing 7.5 ml of distilled water and 0.5 ml of Folin-Ciocalteu phenol reagent, 1 ml of 35 % Na2CO3 solution and dilute to 10 ml with distilled water. The mixture was shaken well and kept at room temperature for 30 min. A set of reference standard solutions of Catechin (12.5, 25, 50, 100 and 200 μg/ml) were prepared. Absorbance for test and standard solutions were measured against the blank at 725 nm with an UV/Visible spectrophotometer. The tannin content was expressed in terms of mg of CE /g of extract.
- Total antioxidant activity
The antioxidant activity is determined by the conjugated diene method (Lingnert et al., 1979). Each extract (0.1 - 20 mg/ml) in water or ethanol (100 μl) is mixed with 2.0 ml of 10 mM linoleic acid emulsion in 0.2 M sodium phosphate buffer (pH 6.6) in a test tube and kept in dark at 37°C to accelerate oxidation. After incubation for 15 h, 0.1 ml from each tube is mixed with 7.0 ml of 80% methanol in deionized water and the absorbance of the mixture is measured at 234 nm against a blank in a spectrophotometer. The antioxidant activity is calculated as follows:
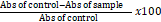
Ascorbic acid, (Lingnert et al., 1979) was used as a positive control.
- Evaluation of in vitro Antioxidant Potentials of ADL
- Assay of DPPH Radical Scavenging Capacity of the Extract
The free radical scavenging ability of the extracts against DPPH (1,1-diphenyl-2 picrylhydrazyl) free radical was evaluated as described by Gyamfi et al. (1999). Briefly, appropriate dilution of the extracts (1 ml) was mixed with 1 ml, 0.4 mmol/L methanolic solution containing DPPH radicals, the mixture was left in the dark for 30 min and the absorbance was taken at 516 nm. The DPPH free radical scavenging ability was subsequently calculated as:
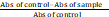
X 100
- Assay of CUPRAC of the Extract
In order to determine the cupric ions (Cu2+) reducing ability of ADL, the method proposed by Apak et al. (2004) was used. In this assay, 0.01M of CuCl2 solution, 7.5mM of ethanol neocuproine solution, and 1.0M of CH3COONH4 buffer solution were added to each test tube containing different concentrations of standard antioxidant (trolox) or extracts, respectively. Finally, total volume was adjusted to 2ml with distilled water and incubated for 30 minutes at room temperature. Absorbance was measured at 450 nm against a reagent blank. Increased absorbance of the reaction mixture shows increased reduction capability of solution. Trolox can be used as a positive control.
- Hydroxyl radical scavenging (OH) assay
The scavenging ability for hydroxyl radicals is measure by the method of Kunchandy and Rao (1990). The reaction mixture (1.0 ml) consist of 100 μl of 2-deoxy-Dribose (28 mM in 20 mM KH2PO4 -KOH buffer, pH 7.4), 500 μl of the extract, 200 μl EDTA (1.04 mM) and 200 μM FeCl3 (1:1 v/v), 100 μl of H2O2 (1.0 mM) and 100 μl ascorbic acid (1.0 mM) which is incubated at 37ºC for 1hour. 1.0 ml of thiobarbituric acid (1%) and 1.0 ml of trichloroacetic acid (2.8%) are added and incubated at 100ºC for 20 min. After cooling, absorbance is measured at 532 nm, against a blank sample. Mannitol, (Kunchandy and Rao, 1990), can be used as a positive control. 2.4.4.3 Metal chelating activity
The chelation of ferrous ions is estimated using the method of Dinis et al. (1994). 0.1 ml of the extract I added to a solution of 0.5 ml ferrous chloride (0.2 mM). The reaction is initiated by the addition of 0.2 ml o ferrozine (5 mM) and incubated at room temperature for 10 min and then the absorbance is measured at 562 nm. EDTA (Dinis et al., 1994) was used used as a positive control.
- Nitric oxide radical scavenging activity
Various concentrations of ADL (6.25-200 µg/ml) and sodium nitroprusside (5mM) in phosphate buffer saline (0.025 M, pH 7.4) in a total volume of 3 ml was incubated at room temperature for a period of 150 min. After which, 0.5 ml of the incubated solution and 0.5 ml Griess’ reagent (1% sulphanilamide, 2% O-Phosphoric acid and 0.1% naphthyethylene diamine dihydrochloride) were added together and allowed to react for 30 min. Control samples without the test compounds but with equal volume of buffer was prepared in a similar manner as done for the test. The absorbance of the chromophore formed during diazotisation of nitrite with sulphanilamide and successive coupling with naphthyethylene diamine dihydrochloride was measured at 546 nm. The experiment was carried out using Quercetin as positive control (Rao et al., 2010). The percentage inhibition of the extract and standard was calculated as:
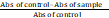
X 100
2.4.4.5 In vitro lipid peroxidation
The ability of ADL to inhibit lipid peroxidation in tissues was determined by measuring the level of thiobarbituric acid reactive substances (TBARS) as described by Puntel et al. (2005).Aliquots of brain homogenate (100 µl) was incubated at 37oC in a water bath in the presence of 20 µl of different concentrations of ADLE (25, 50, 100, 200 and 400 µg/ml) and 30 µl of the respective prooxidant (Fe2+ or sodium nitroprusside, SNP). The colour was developed by adding 200 µl of 8.1% SDS (sodium dodecyl sulphate) to the reaction mixture containing the tissue homogenates. This was subsequently followed by the addition of 500 µl of acetic acid/ HCl buffer (pH 3.4) and 500 µl 0.6% thiobarbituric acid (TBA). The mixture was heated at 100oC for 1 h. The levels of TBARS produced were measured at 532 nm. The absorbance was compared with malondialdehyde (MDA) standard curve.
2.5 In vivo Antioxidant evaluation
2.5.1 Grouping and treatment of experimental animals
Male wistar albino rats weighing 190 ± 20 g were divided into seven groups of seven animals per group. Triton X-1339 was dissolved in normal saline. All animals were fasted for 18 hours before commencing the treatment for 14 days.
Group I: Normal saline (Control)
Group II: Triton (200 mg/kg b.w.)
Group III: Triton (200 mg/kg b.w.) + Anthocleista djalonensis (200 mg/kg b.w.)
Group IV: Triton (200 mg/kg b.w.) + Anthocleista djalonensis (400 mg/kg) b.w.)
Group V: Triton (200 mg/kg) b.w.) + Anthocleista djalonensis (600 mg/kg) b.w.)
Groups II –V were fasted for 24 h prior to Triton administration. ADL was administered 24 hour after triton induction once daily for 14 consecutive days.
- Sacrifice, harvesting of organs and preparation of tissue homogenate from experimental animals
Rats were sacrificed by cervical dislocation. Heart and liver tissues were excised and rinsed in cold 0.9% NaCl solution, blotted with filter paper and weighed. The tissues were then minced with scissors in 10 volumes of phosphate buffer saline, pH 7.4 and homogenized in a teflon homogenizer. The homogenates were later centrifuged at 10000 g for 15 minutes at 4oC and the supernatants, termed the post mitochondrial fractions (PMF) were collected, stored at 4°C until needed for the enzyme assays. The clear supernatant was used to evaluate the in vivo antioxidant assay.
2.5.3 Cardiac and hepatic antioxidant status
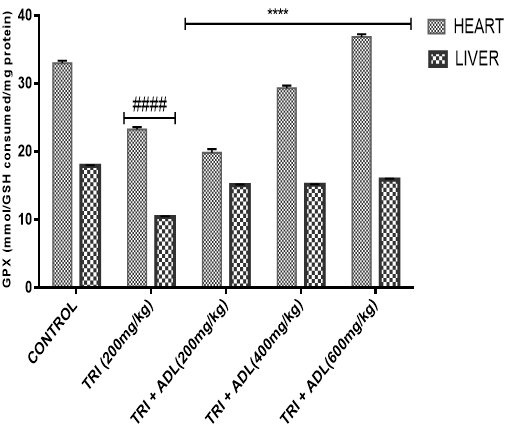
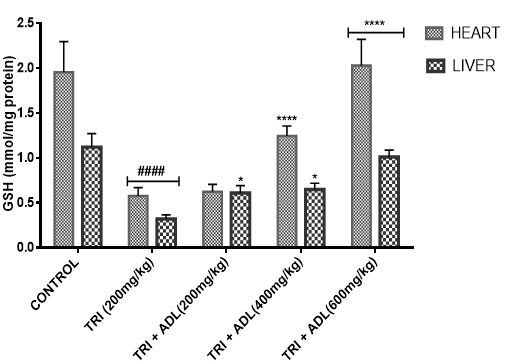
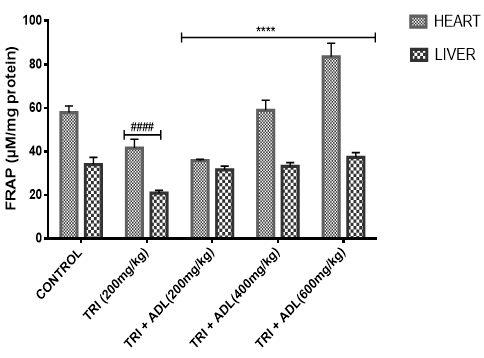
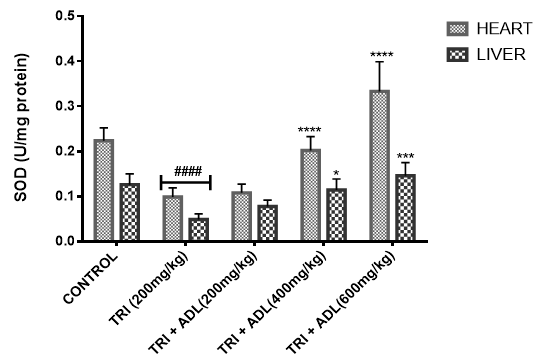
Activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), reduced glutathione (GSH), peroxidation and ferric reducing antioxidant power (FRAP) of hepatic and cardiac cells were measured.
2.6 Statistical Analysis
Results were analysed using appropriate analysis of variance (ANOVA) followed by Tukey multiple comparison tests. In all the tests, p<0>
3.0 Results
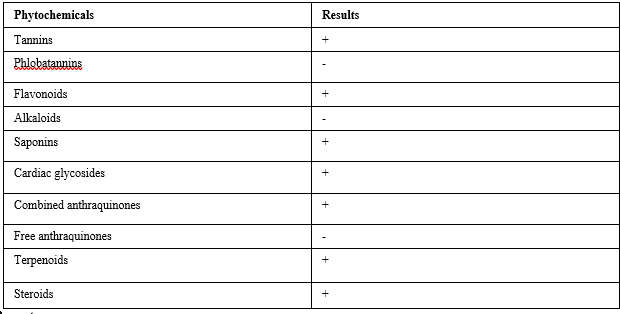
Phytochemical constituents of Anthocleista djalonensis leaf extract
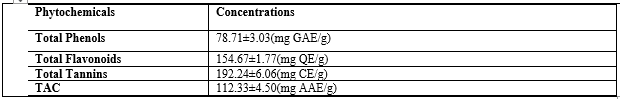
The constituents in the aqueous methanol extract of Anthocleista djalonensis leaf (ADL) is presented in Table 1. The results revealed the presence of tannins, flavonoids, saponins, cardiac glycosides, combine anthraquinones, terpenoids and steroids; while phlobatannins, alkaloids and free anthraquinones are absent.
Quantitative Analysis of phytochemicals in Anthocleista djalonensis leaf
Table 2 showed the result of quantitative analysis of ADL. Total Phenolic, flavonoid and tannin contents were present in significant amount (78.71±3.03mg gallic acid equivalent/g extract, 154.67±1.77mg quercetin equivalent/g extract and 192±6.06mg catechin equivalent/g extract), respectively. The total antioxidant capacity was 112±4.50mg ascorbic acid equivalent/g extract.
In vitro antioxidant activity of methanolic leaf extracts of Anthocleista djalonensis
3.3.1 DPPH radical scavenging activity of methanolic leaf extracts of Anthocleista djalonensis
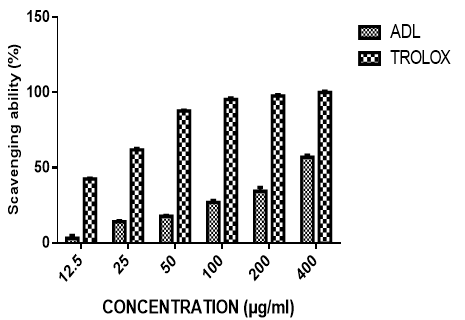
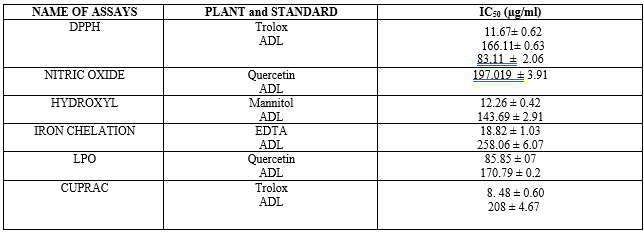
The ability of the extract to scavenge the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and the reference trolox is presented in figure 1. The extract exhibited concentration-dependent inhibition of DPPH radical with an IC50 value of 166.11± 0.63µg/ml. This was higher than that of trolox with an IC50 value of 11.67± 0.62µg/ml.
Nitric oxide scavenging activity of methanolic leaf extracts of Anthocleista djalonensis
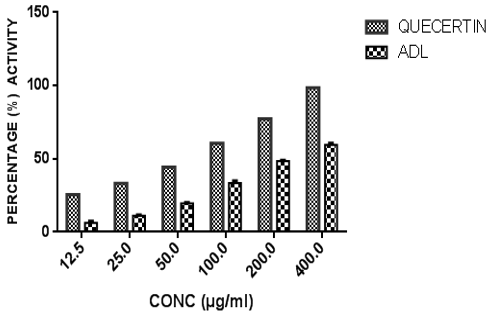
Figure 2 shows the nitric oxide (NO) scavenging activity of ADL in comparison with a standard antioxidant, quercetin. The extract exhibited concentration-dependent scavenging ability against NO radical with an IC50 value of 197.02± 3.91 µg/ml. This was higher than that of quercetin with an IC50 value of 83.11 ± 2.06 µg/ml. At the highest concentration tested (400 μg/ml), the percentage NO radical scavenging activity for ADL and quercetin were 59.54%, 98.45% respectively
Hydroxyl radical scavenging activity of methanolic leaf extracts of Anthocleista djalonensis
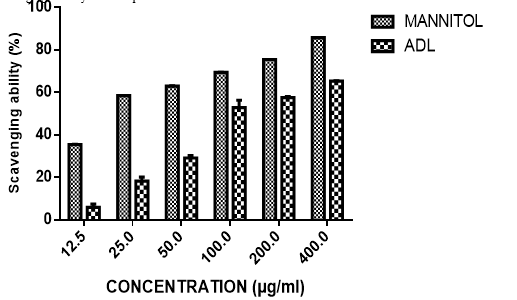
The capability of Anthocleista djalonensis leaf extract to scavenge the hydroxyl radicals generated by the presence of 1, 10-phenanthroline (OH) radical as compared to the reference Mannitol is shown in Figure 3. The hydroxyl radical (OH) scavenging activity of ADL as presented shows that the extracts had a concentration dependent scavenging activity. The extract showed appreciable scavenging activity that compared well with Mannitol. At the highest concentration tested (400 μg/ml), the percentage OH radical scavenging activity for ADL and Mannitol were 65.41%, 85.75% respectively. The extract exhibited concentration-dependent decrease in OH radical with an IC50 value of 143.69 ± 2.91µg/ml and Mannitol 12.26 ± 0.42µg/ml
Iron chelating activity of methanolic leaf extracts of Anthocleista djalonensis
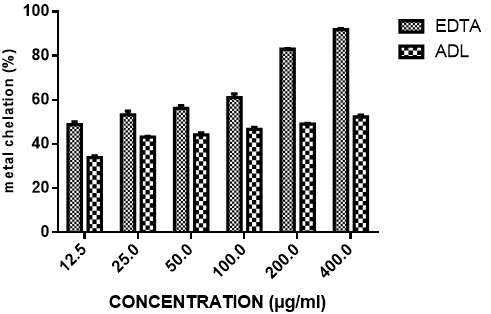
Iron chelating activity of ADL is presented in Figure 4 and compared with EDTA. The result revealed that extract of ADL has concentration-dependent Fe (II) chelating activity with over 50% chelation at 400µg/ml extract concentration. This was however significantly lower than that of the standard EDTA with about 90% chelation also at 400µg/ml concentration. The iron chelating activity of the extract is not significantly concentration dependent. At the highest concentration tested (400 μg/ml), the percentage of iron chelating activity for ADL and EDTA were 52.33%, 91.86% respectively. IC50 of ADL and EDTA were 258.06 ± 6.07µg/ml and 18.82 ± 1.03 µg/ml respectively.
Lipid peroxidation inhibitory activity of methanolic leaf extracts of Anthocleista djalonensis
Figure 5 shows the ability of ADL to inhibit lipid peroxidation in comparison with ascorbic acid. The extract demonstrated concentration dependent activity and compared favourably with ascorbic acid at lower concentrations but was not so at higher concentrations (100µg/ml-400µg/ml). At the highest concentration tested (400 μg/ml), lipid peroxidation activity for ADL and ascorbic acid were 58.82%, 87.96% respectively. The results further revealed that incubation of tissues in the presence of extract resulted in decrease and concentration-dependent inhibition of lipid peroxides accumulation caused by Fe (II). The extract and ascorbic acid showed IC50 values of 170.79 ± 0.2 µg/ml and 85.85 ± 0.7 µg/ml for the inhibition of Fe (II) peroxidation
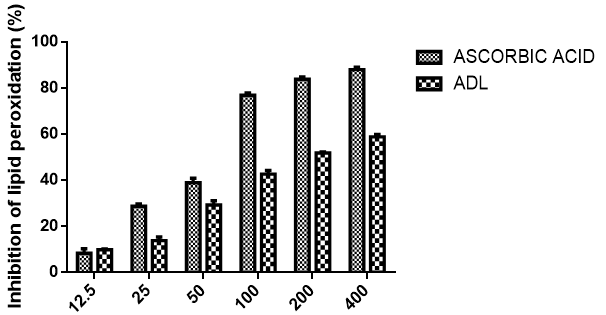
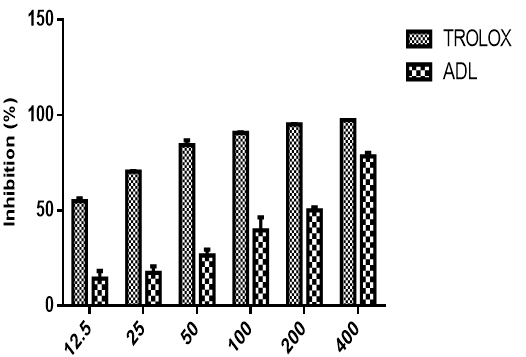
Figure 6 shows the cupric ion reducing antioxidant capacity of methanolic leaf extract of Anthocleista djalonensis in comparison with Trolox. The extract demonstrated concentration dependent activity and compared favourably with Trolox at highest concentrations but was not so at lower concentrations (12.5µg/ml-200µg/ml). At the highest concentration tested (400 μg/ml), the cupric ion reducing antioxidant capacity of ADL and trolox standard were 78.49%, 97.45% while the IC50 were 208.31 ± 4.67 µg/ml and 8.48 ± 0.60 µg/ml respectively
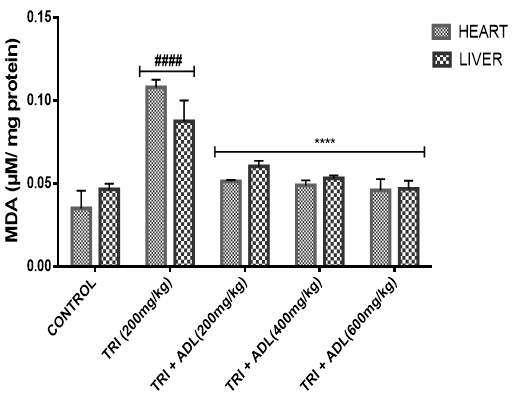
Effect of Methanolic Leaf Extract of Anthocleista djalonensis on Cardiac and hepatic Levels of Oxidative Stress Markers in Wistar Albino Rat
In the present study, triton toxicity in rats resulted in oxidative stress as evidenced by the reduction in myocardial and hepatic antioxidant enzyme activities (GPx, FRAP, catalase, and SOD), myocardial and hepatic GSH level, and an increase in TBARS levels. These anomalies were prevented upon post treatment with ADL which returned both the elevated cardiac and hepatic MDA to normal control values, and restored the altered levels of antioxidant indices. The effect of Anthocleista djalonensis extract on the antioxidant status of triton-treated rats suggests a dose influence with the highest protective effects at 600 mg/kg. The results showed that ADL compared well with rosuvastatin in reversing the oxidative stress caused by triton (figure 7-11).
Discussion
Before the advent of modern medicine herbs and herbal products were the mainstay of treatments around the world (Shi et al., 2010). The phytochemicals present in commonly consumed plant foods and medicinal plants are normally non-toxic and have the potential for preventing chronic diseases (Eddouks et al., 2014). Plant extracts often contain high concentration of flavonoids and phenolic compounds. As potent antioxidants, flavonoids are especially important for protection against human diseases. It has been widely accepted that biological values of plants depends on their bioactive components such as Saponins, phenols, flavonoids, diterpenes, tannins, alkaloids, steroids and other phytochemicals (Veermuthu et al., 2006). The multiple properties of these phytochemicals have made them more attractive, as they can modulate various aspects of diseases like lipid peroxidation involved in diabetes and a variety of disease conditions (Tiwari, 2001; Olaleye et al., 2006). Phytochemical analysis of Anthocleista djalonensis leaf extract (ADL) showed different phytoconstituents viz. glycosides, tannins, flavonoids, saponins, anthraquinones, terpenoids and steroids. Plants contain a wide variety of free radicals scavenging molecules including phenols, flavonoids, vitamins, terpenoids that are rich in antioxidant activity (Madsen and Bertelsen, 1995; Cai and Sun, 2003). Many plants, citrus fruits and leafy vegetables are the source of ascorbic acid, vitamin E, caratenoids, flavanols and phenolics which possess the ability to scavenge the free radicals in human body. Alkaloids have been reported to have pharmacological effects on human and animals (Tiburski et al., 2011). Tannins which are phenolic compounds that precipitate proteins with their biological and pharmacological effects are known to have antibacterial, antiviral, enzyme inhibition, antioxidative, antimutagenic and antitumoral properties (Pathak et al., 2010). Significant antioxidant properties have been recorded in phytochemicals that are necessary for the reduction in the occurrence of many diseases (Hertog and Feskens, 1993; Anderson & Teuber, 2001). Many dietary polyphenolic constituents derived from plants are more effective antioxidants in vitro than vitamins E or C, and thus might contribute significantly to protective effects in vivo (Rice-Evans and Miller, 1997; Jayasri et al., 2009). Steroids and flavonoids groups of phenolic compounds with antioxidant activities are found in varying amounts in foods and medicinal plants which have been shown to exert potential antioxidant activities by contributing to the antioxidant defense system (Olaleye and Adegboye, 2006). The phytoconstituents which are detected in ADL could be partly responsible for the observed antioxidant activity of the extracts. Quantitative analysis of phytochemicals revealed total Phenolic, flavonoid and tannin contents were present in significant amount (78.71±3.03mg gallic acid equivalent/g extract, 154.67±1.77mg quercetin equivalent/g extract and 192±6.06mg catechin equivalent/g extract), respectively. The total antioxidant capacity was 112±4.50mg ascorbic acid equivalent/g extract. Phenols have been reported to have various physiological functions including antioxidant, antimutagenic, antitumor and free radical scavenging activities (Podsedek, 2007). Alkaloids and saponins are known to elicit antimicrobial abilities and defend plants against microbial and pathogenic attacks (Sczkowski et al., 1988). Their analgesic, anti-inflammatory, antihypertensive and anti-microbial properties had been reported (Sofowara, 1993). Flavonoids are well known for their antioxidant activity, protecting human, animal and plant cells against the damaging effects of free radicals. Due to this remarkable property including their anti-inflammatory and hypolipidemic, they are being used in numerous medical treatments associated to cancer-prevention and cardiovascular system protection, including prevention of oxidative damage (Chu et al., 2000; Birt et al., 2001).
In this study the in vitro antioxidant activity of Anthocleista djalonensis was performed using different methods namely DPPH, nitric oxide, hydroxyl radical, metal chelation, CUPRAC and lipid peroxidation. These methods are distinguished by their mechanism of actions and are complementary to the study of the antioxidant potential of Anthocleista djalonensis. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) is a stable free radical. On accepting hydrogen from a corresponding donor, its solutions lose the characteristic deep purple colour. DPPH is very popular for the study of natural antioxidants (Villano et al., 2007). It is usually used to estimate the in-vitro antioxidant activity of natural compounds or plant extracts (Philips et al., 2010). As shown in figure 1 the DPPH scavenging activity of methanolic extract of Anthocleista djalonensis leaves compared moderately with trolox standard at all the concentrations tested. The result shows that though the trolox which was used as standard showed a better DPPH scavenging ability than the extract at all the doses tested, the extract still showed appreciable activity in this regard. At the highest concentration of 400μg/ml the extract showed 57.12% inhibition while the standard antioxidant showed 99.00% inhibition of DPPH radical. The result also showed that the extract exhibited a dose-dependent inhibition of DPPH radical. The IC50 of ADL and trolox standard were 166.11µg/ml and 11.67 µg/ml. Low IC50 value showed better antioxidant ability. Nitric oxide is important in inflammatory processes but at an increased level is directly toxic to tissues resulting in vascular damage and other ailments. It is a potent pleiotropic inhibitor of physiological processes such as smooth muscle relaxation, neuronal signaling, and inhibition of platelet aggregation and regulation of cell mediated toxicity (Hagerman et al., 1998). It would be interesting to develop potent and selective inhibitors of NO for potential therapeutic use (Hepsibha, 2010). Figure 2 showed that ADL showed a dose-dependent increase in nitric oxide scavenging activity. The result also shows that though the quercetin which was used as standard showed a better NO scavenging ability than the extract at all the doses tested, the extract still showed appreciable activity in this regard. At 400μg/ml which was the highest concentration tested the extract showed 59.54% scavenging activity while quercetin which was used as standard showed 98.45% nitric oxide scavenging activity. The IC50 of ADL and quercetin standard were 197.02 µg/ml and 83.11µg/ml. Low IC50 value showed better antioxidant ability. The hydroxyl radical is an extremely reactive free radical formed in biological systems and has been implicated as a highly damaging species in free radical pathology, capable of damaging almost every molecule formed in living cells. Hydroxyl radicals are also known to initiate peroxidation of lipid membranes (Halliwell, 1991). The radical has the capacity to form adducts with nucleotides in DNA and cause strand breakage which contributes to carcinogenesis, mutagenesis and cytotoxicity (Thirunavukkarasu et al., 2011). Our result in figure 3 shows that methanolic extract of ADL has a high hydroxyl radical scavenging activity which compared moderately with mannitol in a dose-dependent manner. At the highest concentration tested the extract and standard showed percentage hydroxyl radical scavenging activity of 65.41% and 85.75% respectively. The IC50 of ADL and mannitol standard were 143.69µg/ ml and 12.26 µg/ml. Low IC50 value showed better antioxidant ability. Fe (III) reduction is often used as an indicator of electron donating activity, which is an important mechanism of phenolic antioxidant action (Nabavi et al., 2009a). The reducing ability of a compound generally depends on the presence of reductones (antioxidants), which exert the antioxidant activity by breaking the free radical chain by donating a hydrogen atom (Meir et al., 1995). Iron is essential for life as it is required for oxygen transport, respiration and for activity of many enzymes. Chelating agents inhibit lipid peroxidation by stabilizing the transition metals. Decrease in the red color ferrozine-Fe2+ complex indicates high radical scavenging activity of the compound. Figure 4 shows the percentage iron-chelating activity of our extract. The result shows that though the EDTA which was used as standard showed a better iron-chelating ability than the extract at all the doses tested, the extract still showed appreciable activity in this regard. At 400 μg/ml the extract and standard showed 52.33% and 91.86% iron-chelating activity respectively. The IC50 of ADL and EDTA standard were 258. 06µg/ ml and 18.82 µg/ml. Low IC50 value showed better antioxidant ability. Lipid peroxidation in biological systems has long been thought to be a toxicological phenomenon that can lead to various pathological consequences (Hochestein and Attalah, 1988). Lipid peroxidation is caused by the generation of free radicals from a variety of sources including organic hydroperoxides, redox cycling compounds and iron-containing compounds. The TBARS assay has been used to measure the degree of lipid peroxidation. TBA reacts specifically with malondialdehyde (MDA), a secondary product of lipid peroxidation to give a red chromogen, which may then be determined spectrophotometrically (Giri et al., 2010). In this study, it was observed that methanolic extract of ADL showed a moderate inhibition of lipid peroxidation. The result as presented in figure 5 shows that the extract has a dose dependent inhibition of lipid peroxidation with the highest concentration tested at 400μg/ml showing a percentage inhibition of 58.82% while ascorbic acid had a percentage inhibition of 87.96% at the same concentration. The IC50 of ADL and ascorbic ascid standard were 170.79µg/ ml and 85.85 µg/ml. Low IC50 value showed better antioxidant ability. Copper ion reducing antioxidant capacity assay utilizes the copper (II) neocuproine reagent as the chromogenic oxidizing agent. This method is more advantageous over other ET based assays as the working pH range for this assay is the physiological pH (7) in contrast to alkaline pH used in Folin method or acidic pH used in FRAP method. It is applicable to both hydrophilic and lipophilic antioxidants (unlike folin and DPPH assays), has a selective action on antioxidant compounds without affecting sugars and citric acid commonly present in foodstuffs and has a capacity to assay –SH bearing antioxidants (unlike FRAP) (Apak et al., 2007). The CUPRAC assay method describes the development of a simple and widely applicable antioxidant capacity assay for flavonoids, phenolic acids, hydroxycinnamic acids, thiols, synthetic antioxidants and vitamin C and E (Apak et al., 2013). Our result as presented in figure 6 shows that the extract has a dose dependent CUPRAC activity with the highest concentration tested at 400μg/ml showing a percentage inhibition of 78.49% while ascorbic acid had a percentage inhibition of 97.45% at the same concentration. The result shows that though the trolox which was used as standard showed a better CUPRAC activity than the extract at all the doses tested, the extract still showed appreciable activity in this regard. The IC50 of ADL and ascorbic acid standard were 208.31µg/ ml and 8.48 µg/ml. Low IC50 value showed better antioxidant ability. Anthocleista djalonensis leaf (ADL) extract demonstrated free radical scavenging ability in a concentration-dependent manner. It may thus be hypothesized that ADL reduces the radicals to their corresponding stable products which are hydrazine and ABTS when it reacts with hydrogen donors in the antioxidant principle. The free radical-scavenging activity of flavonoids is attributed to their hydrogen-donating ability (Manach et al., 2004). ADL extract possesses considerable reducing properties as demonstrated by its ability to reduce Fe3+ to Fe2+. It can thus be assumed that the polyphenolics present in the leaves could act as reducing agents by donating electrons to free radicals and terminating the free radical mediated chain reactions. The presence of reductones has been shown to impart antioxidant action by breaking the free radical chain by the donation of hydrogen atoms. The presence of reductones (i.e. antioxidants) in the sample extracts might cause the reduction of Fe3+/Ferric cyanide complex to ferrous form which can be monitored by spectrophotometrically at 700 nm (Nair et al., 2012). The reducing power of the extract is concentration dependent, which served as a significant indicator of its potential antioxidant activity. Iron can promote lipid peroxidation by decomposing lipid hydroperoxides into peroxyl and alkoxyl radicals that can perpetuate the chain reaction and by means of the Fenton reaction. Metal chelating agents reduce the concentration of metal ions in the Fenton type reaction and thus would protect the system from oxidative damage through inhibition of metal dependent processes (Ak and Gulcin, 2008). Anthocleista djalonensis leaf extract was not as potent as the standard EDTA in iron chelation though its activity was almost like the EDTA standard; it however did show chelating activity by decreasing the colour formation in a concentration-dependent manner. In the current study, the inhibitory effect of ADL against iron induced lipid peroxidation was assessed. Lipid peroxidation is a free radical-mediated process involving lipid-derived radicals, such as alkoxyl and peroxyl radicals, wherein oxidative damage is propagated to polyunsaturated fatty acids. The toxicity of Fe (II) proceeds via the Fenton reaction where iron catalyses a one-electron transfer reactions that generate reactive oxygen species, such as the OH- from H2O2. Iron is capable of decomposing lipid peroxides leading to the generation of peroxyl and alkoxyl radicals and favoring the propagation of lipid oxidation (Varshney and Kale, 1990). NO is produced by the action of sodium nitroprusside (SNP) in vitro, and is capable of causing neuronal damage in cooperation with other reactive oxygen species (ROS) notably superoxide radical to form peroxyl nitrite radical (Berg et al., 2011). Nitric oxide (NO•) is an important chemical mediator generated by endothelial cells, macrophages, neurons, etc. and is involved in the regulation of various physiological process (Saurabh et al., 2012). Nitric oxides formed during their reduction with oxygen or with superoxides such as NO2, N2O4, N3O4 are very reactive. The excess concentration of NO• will cause diseases in human beings which can alter the structural and functional behavior of many cellular components. Nitrite ions react with Griess reagent and form a purple azo dye. The decrease in the formation of purple azo dye reflects the presence of scavengers in the test compounds (Saurabh et al., 2012). The present research revealed that ADL extract exerts concentration-dependent inhibition of iron-induced peroxidation in tissue homogenates. It is remarkable that on the basis of inhibitory concentrations, the extract afforded protection against iron-induced membrane lipid peroxidation. The protection by Anthocleista djalonensis suggests that the aqueous-methanolic extract may protect the tissue homogenates against toxicities, iron overload and nitric oxide.
Anthocleista djalonensis leaf extract was not as potent as the various standards used for in vitro antioxidant radical activity; it however did show ability to ameliorate the damage caused by these radicals by decreasing their effects in a concentration-dependent manner.
Gas chromatography (GC) and Mass Spectrometry (MS), is used to analyze complex organic and biochemical mixtures (Skoog et al., 2007). The GC-MS instrument consists of two main components. The gas chromatography portion separates different compounds in the sample into pulses of pure chemicals based on their volatility (Oregon State University, 2012) by flowing an inert gas (mobile phase), which carries the sample, through a stationary phase fixed in the column (Skoog et al., 2007). Spectra of compounds are collected as they exit a chromatographic column by the mass spectrometer, which identifies and quantifies the chemicals according their mass-to-charge ratio (m/z). These spectra can then be stored on the computer and analyzed (Oregon State University, 2012). GC-MS analysis of methanolic extract of Anthocleista djalonensis revealed the presence of Butanedioic acid, mono (2, 2-dimethylhydrazide, Theobromine, 3-O-Methyl-d-glucose, 2-Butenedioic acid, 2-methyl-(Z)-, 3-Hydroxybenzaldehyde, Hexanoic acid, 3-hydroxy-, ethyl ester, caryophyllene, N-Hexadecanoic acid, Oleic acid, Phytol, 3,7,11,15-Tetramethyl-2-hexadecen-1-ol, Ricinoleic acid, Hexadecanoic acid methyl ester, Octadecanoic acid methyl ester, 9, 12-Octadecadienoic acid methyl ester, 9-Octadecenoic acid (Z)-methyl ester, 5-Ethyl-4-hydroxy-2-methyl-3(2H)-furanone acetate, Nonacos-1-ene, Squalene, Eicosanoic acid methyl ester, LinoleicS acid trimethylsilyl ester, Beta-Tocopherol, Docosanoic acid methyl ester, Campesterol, Stigmasterol, and 9,19-cyclolanostan-3-ol acetate(3 beta). These compounds consist of a stanol ester, unsaturated fatty acid, a triterpene and a saturated fatty acid. The biological activity of these compounds showed that they have antioxidant, cytotoxic, antihyperlipidemic, anti-inflammatory and prevent lipid peroxidation. It has been reported that plant sterols and stanols are of good therapeutic options for the management of hypercholesterolemia (Weingartner et al., 2011) while triterpenes have been reported to have cytotoxic activity (Zhang et al., 2016). Plasma vitamin A (retinol) concentrations were not affected by plant stanol or sterol esters consumption for up to one year (Katan et al., 2003; Hendriks et al., 2003). Stigmasterol present in bark of Butea monosperma showed decrease in hepatic lipid peroxidation and increase in the activities of catalase, superoxide dismutase and glutathione thereby suggesting its antioxidant property (Panda et al., 2009). Stigmasterol isolated from plants were reported to be involved in the synthesis of many hormones like progesterone, androgens, estrogens and corticoids (Kaur et al., 2011) with several pharmacological prospects such as antiosteoarthritic, antihypercholestrolemic, antitumor, hypoglycaemic, antimutagenic, antioxidant, anti-inflammatory and CNS effects (Marquis et al., 1977; Prestwich et al., 1984; Svoboda et al., 1989; Chowdhury et al., 2003; Gook-Che et al., 2005). As one of the major phytosterols, stigmasterol is included among sterol compounds in the diet having potential to reduce the risk of cardiovascular diseases (Ferrer et al., 2017). Stigmasterol present in bark of Butea monosperma showed decrease in hepatic lipid peroxidation and increase in the activities of catalase, superoxide dismutase and glutathione thereby suggesting its antioxidant property Panda, et al., 2009). Squalene, an isoprenoid from the group of polyphenyl compounds, is an intermediate metabolite in cholesterol synthesis possessing antioxidant, immunostimulating, hypolipidemic, cholesterol reducing, anticarcinogenic and anti-inflammatory activity (Kelly, 1999). Squalene is a strong antioxidant due to its large electron exchange capacity without being exposed to molecular disruption. Cardioprotective effect of squalene might be attributed to its antilipidemic, antioxidant, and membrane stabilizing properties (Farvin et al., 2009). N-Hexadecanoic acid, Hexadecanoic acid, ethyl ester, Palmitic acid has the property of antioxidant, hypocholesterolemic, nematicide, pesticide, lubricant activities and hemolytic, 5-alpha is reductase inhibitors (Jegadeeswari et al., 2012; Upgade and Anusha, 2013). N-Hexadecanoic acid has been reported to have activities like being antioxidant (Lalitharani et al., 2009) which is about 68% antioxidant activity (Henry et al., 2002) as well as having cytotoxic activity (Lokesh and Kannabiran, 2017), anti-inflammatory, anti spasmodic and antiviral (Mohammed et al., 2016).Both 9-hexadecenoic acid (Z) - methyl ester and octadecanoic acid methyl ester (Meechaone et al., 2007) have antioxidant activities. Both 9-hexadecenoic acid (Z) - methyl ester and octadecanoic acid methyl ester (Meechaone et al., 2007) have antioxidant activities. Hexadecanoic acid methyl ester and 10-octadecenoic acid methyl ester seem to have the ability to decrease blood cholesterol (Hema et al., 2011). Hexadecanoic acid methyl ester inhibits the cyclooxygenase II enzymes and, thus, produces a selective anti-inflammatory action (Hema et al., 2011). It is believed that the plant phytosterols, unsaturated fatty acids and other phytochemicals detected in methanolic extract of Anthocleista djalonensis may contribute in part to their significant antioxidant properties. Living tissues are endowed with innate antioxidant defense mechanisms through enzymatic and non enzymatic antioxidants that are involved in the quenching of superoxide anions and H2O2 (Halliwell, 2000). CAT, SOD and GPx are antioxidant enzymes which play a significant role in the cellular defense against deleterious effects of free radicals. A decrease in the activities of these enzymes could increase the availability of O2–, NO and H2O2, which can lead to the production of OH radicals, subsequently initiating oxidative damage. The reduced antioxidant activities as well as CAT, SOD and GPx activities in triton toxified hamsters indicated possible oxidative stress conditions, leading to increased levels of O2–, NO and H2O2 and leading to deleterious effects such as loss of integrity and function of cell membranes (Sheela and Augusti, 1995). CAT and SOD had been reported to be inactivated by ROS and lipid peroxides (Daniel et al., 1998)
In the present investigation, the mean activities of GPx, GSH, FRAP, CAT and SOD in hepatic and cardiac tissue samples from triton-treated rats were significantly (
Conflict of interest:
The authors declare that there are no conflicts of interest.
References
- Abuh, F.Y., Wambebe, C., Rai, P.P., Sokomba, E.N., (1990). Hypoglycaemic activity of Anthocleista vogelii (Planch) aqueous extract in rodents. Phyther. Res. 4: 20–24.
View at Publisher | View at Google Scholar - Ak, T., Gulcin, I. (2008). Antioxidant and radical scavenging properties of curcumin, Chemico-Biological Interactions. 174(1): 27–37.
View at Publisher | View at Google Scholar - Ampofo, D., (1977). Socio-cultural and medical perspectives of infertility in Ghana. In: Family welfare and development in Africa. International Planned Parent- hood Federation, London.
View at Publisher | View at Google Scholar - Anderson, K.J. and Teuber, S.S. (2001). Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation, biochemical and molecular action of nutrients. Journal of Nutrition. 131: 2837-2842.
View at Publisher | View at Google Scholar - Apak R, et al., (2013). Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 85: 957-998.
View at Publisher | View at Google Scholar - Apak, R., Güçlu, K., Özyürek M. and Karademir, S.E. (2004). J. Agric. Food Chem., 52: 7970–7981.
View at Publisher | View at Google Scholar - Aqil, F., Ahmad, I., and Mehmood, Z. (2006). Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants, Turkish Journal of Biology, 30(3): 177–183.
View at Publisher | View at Google Scholar - B¨uy¨ukokuroˇglu, M.E., G¨ulc¸in, I., Oktay, M., and K¨ufrevioˇglu, O.I. (2001). In vitro antioxidant properties of dantrolene sodium. Pharmacological Research, 44(6) 491–494.
View at Publisher | View at Google Scholar - Baba, H., Usifoh, C.O. (2011). Phytochemical investigation and anti-inflammatory property of ethanol-water extract of the roots of Anthocleista djalonensis A. Chev. (Gentianiaceae) African Journal of Biotechnology 10(34): 6598-6600.
View at Publisher | View at Google Scholar - Bansal, A. K., Bilaspuri, G. S. (2011). Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2011: 1 – 7.
View at Publisher | View at Google Scholar - Berg, R.M.G., Møller, K., Bailey, D.M., (2011). Neuro-oxidative-nitrosative stress in sepsis. Journal of Cerebral Blood Flow and Metabolism 31: 1532–1544.
View at Publisher | View at Google Scholar - Birt, D.F., Hendrich, S., and Wang, W. (2001). Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacology & Therapeutics, 90: 157-177.
View at Publisher | View at Google Scholar - Broadhurst, R.B., Jones W.T. (1978). Analysis of condensed tannins using acidified vanillin. J Sci. Food. Agric. 29: 788-794.
View at Publisher | View at Google Scholar - Burkill, H.M. (1995). The useful Plants of West Tropical Africa, 2nd Ed., Vol. 3, Royal Kew Botanical Gardens. Kew. London. pp: 522-527.
View at Publisher | View at Google Scholar - Cai, Y.Z. and Sun, M. (2003). Antioxidant activity of betalins from plants of the Amaranthacea. Journal of Agriculture and Food Chemistry. 51: 2288-2294.
View at Publisher | View at Google Scholar - Chah, K.F., Eze C.A., Emuelosi, C.E., Esimone, C. O. (2006). Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J. Ethnopharmacol., 104(1): 164-167.
View at Publisher | View at Google Scholar - Chowdhury, R., Rashid, R.B., Sohrab, M.H., C.M. Hasan, (2003). Pharmazie, 58(4): 272-273.
View at Publisher | View at Google Scholar - Chu, Y.H., Chang, C.L., and Hsu, H.F. (2000). Flavonoid contents of several vegetables and their antioxidant activity. Journal of the Science of Food and Agriculture, 80: 561-566.
View at Publisher | View at Google Scholar - Daniel, R.S., Mathew, B.C., Devi, K.S., Augusti, K.T. (1998) Antioxidant effect of two flavonoids from the bark of Ficus bengalensis Linn in hyperlipidemic rats. Indian Journal of Experimental Biology 36(9): 902–906.
View at Publisher | View at Google Scholar - Diallo, A., Traore, M. S., Keita, S. M., Balde, M.A., Keita, A., Camara, M., VanMiert, S., Pieters, L., Balde, A.M., (2012). Management of diabetes in Guinean traditional medicine: an ethnobotanical investigation in the coastal low lands. J. Ethno- pharmacol. 144: 353–361.
View at Publisher | View at Google Scholar - Dinis, T.C.P., Madeira, V.M.C., Almeida, L.M. (1994). Action of phenolic derivatives (acetoaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxy radical scavengers. Arch. Biochem. Biophy. 315: 161-169.
View at Publisher | View at Google Scholar - Droge, W. (2011). Free radicals in the physiological control of cell function. Physiol. Rev. 82: 47–95.
View at Publisher | View at Google Scholar

 Clinic
Clinic
