Research Article | DOI: https://doi.org/10.31579/2835-9232/013
Distribution of Some Potential Pathogenic Microbial Risk Pathogens amongst Pet Dogs in Port Harcourt Metropolis: A Critical Public Health Community Alert
- Amadi, F, O 1
- Nwokah, G E. 1
- Onwuli, D 2
- Reuben, E. 3
- Orabueze, I. C 4
- Azuonwu, G. 5
- Poplong, A.N 5
- Tamuno-Boma, O. 6
- John-Amadi, V. S. 7
- Ekwuozor, I. K 7
- Azuonwu, O 6*
- Vetty Agala 8
1 Department of Medical Laboratory Science, Medical Bacteriology/Virology/Parasitology Unit, Rivers State University, Nkpolu–Oroworukwo, Port Harcourt, Rivers State, Nigeria.
2 Department of Medical Laboratory Science, Chemical Pathology Unit, Rivers State University, Nkpolu–Oroworukwo, Port Harcourt, Rivers State, Nigeria.
3 Department of Human Physiology, College of Medicine, Rivers State University, Nkpolu–Oroworukwo, Port Harcourt, Rivers State, Nigeria.
4 Faculty of Pharmacy, Department of Phrmacovigilence, University of Lagos, Nigeria
5 Departments of Biological Science, Faculty of Science, Federal University of Kashere, Gombe State, Nigeria
6 Department of Biochemistry, Rivers State University, Nkpolu–Oroworukwo, Port Harcourt, Rivers State, Nigeria.
7 Department of Animal and Environmental Biology, Rivers State University, Nkpolu–Oroworukwo, Port Harcourt, Rivers State, Nigeria.
8 Department of Community Medicine, University of Port Harcourt, Choba, Rivers State, Nigeria.
*Corresponding Author: Obioma Azuonwu, Department of Medical Laboratory Science, Medical Bacteriology/Virology/Parasitology Unit, Rivers State University, Nkpolu–Oroworukwo, Port Harcourt, Rivers State, Nigeria.
Citation: Azuonwu, O, Amadi, F, O, Nwokah, G E., Onwuli, D, Reuben, E, et al., (2023), Distribution of Some Potential Pathogenic Microbial Risk Pathogens amongst Pet Dogs in Port Harcourt Metropolis: A Critical Public Health Community Alert, International Journal of Clinical Epidemiology, 2(2); DOI:10.31579/2835-9232/013
Copyright: © 2023 Obioma Azuonwu, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 11 February 2023 | Accepted: 20 February 2023 | Published: 03 March 2023
Keywords: domestic dogs; public health; pathogens; microbial; risk factors; port harcourt
Abstract
The role of pets as one of the most significant transmitters of zoonotic infections and antimicrobial-resistant genes has been overlooked for a very long time, especially among developing countries of the world with weak prospects of healthcare facilities and much needed skilled and well exposed manpower to tackle the menace. However, the most interspecies pathway for the spread of resistant bacteria is closely hinged on the interaction between humans and their pets. This study aimed to determine the distribution of some potentially risky microbial pathogens among pet dogs in Port Harcourt. A cross-sectional study design and a convenient random sampling technique were explored, involving forty-eight (48) dogs, with a total of one hundred and forty-four (144) swab samples, which were collected aseptically from the ears (48), nose (48), and fur (48) respectively. Standard microbiological laboratory techniques were explored to isolate and identify the organisms. However, a total of 127 swab samples yielded microbial growth, while there was no microbial growth in 17 swab samples, with a prevalence of 88.19% and 11.80%, respectively. The number of isolated bacteria was 248 (84.93%) and the number of isolated fungi was 44 (15.07%). These distributions were statistically significant at p < 0.05. The ear swabs contained the fewest number of isolated organisms with 93 (30.03%), followed by nose swabs with 96 (32.88%) and fur swabs with 103 (35.27%). The distribution of bacterial isolates in the dogs did not differ significantly by gender at p > 0.05. Nevertheless, at p < 0.05, the gender of the pet dog owners and the frequency of their veterinary visits had a significant impact on the distribution of pathogens among the pets. Escherichia coli was isolated in 60 samples (20.55%) being the most prevalent bacterial organism, followed by Staphylococcus aureus in 48 samples (16.41%), while Proteus mirabilis and Streptococcus agalactia had the lowest incidence with only one isolate (0.34%) among the 14 bacteria species isolated. Among the 11 fungal organisms isolated, Candida albicans was the most prevalent with 13 isolates (4.45%), followed by Candida species with 12 isolates (4.11%), and three Trichophyton species, namely Trichophyton kragdeni, Trichophyton carious and other Trichophyton species were the least prevalent with 1 isolate (0.34%) each. These observations were statistically significant at p < 0.05. Ear swabs and fur swabs showed the highest prevalence of fungal infections with 17 isolates (5.82%) each, whereas nose swabs had the lowest prevalence with 10 fungal isolates (3.43%). In conclusion, it was observed that pet dogs harbour important risky pathogens of public health concern in their nostrils, skin, and ears, and the fact that these domestic dogs are kept as pets in homes makes them even more dangerous. Therefore, proper public health education regarding the risks associated with dog ownership, as well as the need for regular vaccination and proper hygiene when handling these animals, must be strongly underpinned and sustained, so as to protect the overall goals of public health protection in our urban and remote communities.
Introduction
The role of pets as one of the most notable and critical sources of antimicrobial-resistant bacteria has been overlooked for a very long time. They are also primary sources of antimicrobial-resistant pathogens littered in our environment through their interaction with the ecosystem and have been considered to be part of food-chain producing and poisoning pathogens. Multidrug-resistant (MDR) bacteria are most effectively transferred between species through close contact between humans and their pets [1]. When dogs and cats share a home with their owners, they are exposed to the same surfaces, foods, and objects, which increase the likelihood of antimicrobial resistance spreading potency. Thus, this makes it very difficult to treat infections with a high probability of anti-microbial resistance genes from zoonotic origins. Furthermore, advances in veterinary medicine and a growing sense of social responsibility for the welfare and health of pets have increased the pets' life expectancy, resulting in an increase in demand from the number of geriatric patients who require regular antimicrobial therapy, since they are frequently exposed to chronic diseases or immunocompromised conditions [2]. These domestic pets can be colonised or infected with a wide variety of pathogenic microbes that cause zoonotic diseases in both animals and humans [3]. Thus, these can be directly or indirectly transmitted to humans respectively. Nonetheless, it is strongly believed that the animal serves as a reservoir for zoonotic infections in most cases, whereas in the second option, it may merely act as a mechanical vector during the transmission mechanism process, as reported by Karesh et al., [4]. Nevertheless, directly or indirectly, animals play a critical role in the transmission, spread, and maintenance of numerous diseases in animal and human populations, as also supported by the by WHO [5] report.
Nevertheless, zoonotic bacterial infections that are associated with pets have received less attention, unlike food-borne zoonotic diseases that have always been on the front burner of news and investigations across the globe. However, increased close contact between household pets and humans creates favourable conditions for the transmission of bacterial zoonoses, either through petting, licking, or physical injuries, or indirectly through contaminated food and the environment. Those with compromised immune systems, including the young, the elderly and pregnant women should be of particularly great concern. Young children are increasingly susceptible to zoonotic epidemic outbreak infections, due to their lack of hygienic practices and close personal contact with these animals and household environments such as floors and carpets [6]. The primary modes of transmission for bacterial zoonoses are through physical injuries (bites and scratches), inhalation (Psittacosis), contact with urine or urine-contaminated environments, and faecal-oral ingestion through the mouth [7; 8]. Stall et al.,
[9] strongly opined that household pets are significant sources of zoonotic pathogenic infections, simply due to the close and intimate interactions between the household environment and these supposedly animals in question. Nevertheless, many people are probably and generally unaware of the massive public health risks, that are undoubtedly associated with interacting with these animals and also may not be aware of how to reduce these risks through the firm application of personal hygiene and safety strategies of international best practices [9 ;10]. Nonetheless, against the above backdrop, there is a visible paucity of data/robust research information on the above subject matter in South-South part of Nigeria thus; it is firmly believed that data generated through this study would probably form baseline research information on the distribution of potential risky pathogens that are linked to domestic pet Dogs. Furthermore, it would also underpin the researcher’s huge understanding of the prevailing prevalence of the emerging zoonotic diseases, that are associated with pet dogs and possible risk factors that are promoting the trend in the region.
Methodology
Study Area: This research was conducted in Port Harcourt, which is the capital of Rivers State. Rivers State is the sixth most populous state in Nigeria, with over five million inhabitants [11]. It is located in the Niger Delta at 4°451N6°5O1E/4.750°N6.833°E [11] and is rich in rainforests and mangrove swamps. Port Harcourt is the largest and most densely populated city in Rivers State, as well as the economic centre of Nigeria's petroleum industry [11]. As a result of this fact, many communities within the state are oil-producing communities. The city has been dubbed "The goose that lays the golden egg" in reference to a large number of multinational corporations present in the city. Given this reason, many migrants from far and near seek greener pastures in the region.
Study Population/Design/Eligibility Criteria: The study population comprised pet dogs in Port Harcourt,
Rivers State. The study design was cross-sectional. The inclusion criteria included Pet dogs living as household pets and those whose owners agreed to participate in the study. However, the exclusion criteria include those who could not give their consent to participate in the study and none household pet dogs.
Sampling Technique: The sampling technique used was a convenient random sampling research technique, involving a total of 48 dogs from which (48) nose swabs, (48) ear swabs and (48) fur swabs were collected, giving a total of 144 swab samples which were collected aseptically.
Sample collection and processing
: The sample type comprised swabs collected from the nose, ear and fur of pet dogs. Samples were collected using sterile cotton wool swabs which were kept on ice parks to prevent or reduce deterioration during transportation time back to the laboratory for analysis, however, culture and analysis of each batch of samples were done on the same day they were collected. All bacterial pathogens were isolated using MacConkey agar, Blood agar, and Chocolate agar and Saboroud agar plates, incubated overnight at 370C while plates that showed no growth were re-incubated for 48 hours at the same temperature except MacConkey plates. All fungal isolates were isolated from the Saboroud plates. The cultural characteristics of the isolated pathogens were identified using microbiological identification key and biochemical tests matched with standard fungal plates on the internet key alongside microbiological microscopic appearance using potassium hydroxide (KOH). However, candida species tube tests were carried out on all suspected Candida colonies using fresh plasma as described by Cheesborough, [12]. The sample analysis was done in the microbiology department of University of Port Harcourt Teaching Hospital, Choba, which is a tertiary healthcare facility with robust scientific infrastructure.
Data Analysis
Data analysis was done using SPSS (Statistical Package for Social Sciences,) version 21 with p-value set at 0.05
Level of significance. Results of the analysis such as t-test, and chi-square/cross-tabulation analysis were presented in graphs, histograms, tables and bar charts respectively.
Results
Table 1 shows the infection prevalence of the different types of sample swab sites explored in the study, the result showed that ear swabs had the least number of positive cases at 41 (28.47%) while the fur and nose swabs stood at 43 (29.86%) positive cases each respectively. Also, the results of the frequency and percentage distribution of some bacterial and fungal isolates as shown in table 2 indicate that fur (103) had the highest number of bacterial isolates seconded by the nose swab (96) and the least was ear (93), however, the result of the number of fungi pathogens isolated showed that the highest was isolated from the ear and fur (17 each) while the nose had the least (10).
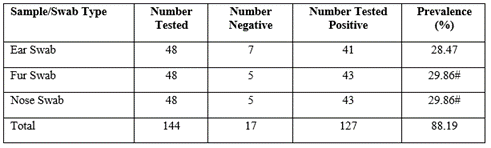
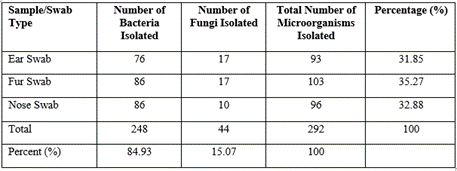
Table 3 illustrates the frequency and percentage occurrence of Bacteria from different samples. Proteus mirabilis and Streptococcus agalactia had the least prevalence of 0.34%, while Escherichia coli was most prevalent (20.55%) followed by staphylococcus aureus with a prevalence of 16.44%. other microorganisms isolated had prevalence rates as follows; Bacillus species (13.70%), Staphylococcus species (14.38), Klebsiella species (6.16%), Staphylococcus epidermidis (4.45%), Serratia mercescenece (2.39%), Streptococcus faecalis (0.60%), Streptococcus species (2.74%), Pseudomonas aeruginosa (0.68%) and Pseudomonas species (1.03%) respectively.
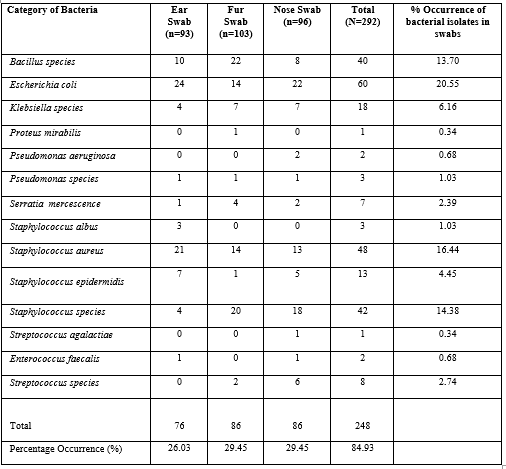
Table 3: Frequency and Percentage Occurrence of Bacterial Isolates from Pet Dog
Table 4 shows the frequency and percentage occurrence of fungal pathogens isolated from the different samples. Candida albicans was the most prevalent fungal isolate (4.45%). This was closely followed by Candida species with a prevalence of 4.11%. The least prevalence was observed among three different Trichophyton species including Trichophyton kragdeni (0.34%), Trichophyton carious (0.34%) and other Trichophyton species (0.34%). Other fungi species isolated from this study and their prevalence rates are as follows; Aspergillus fumigatus (1.03%), Aspergillus niger (1.37%), other Aspergillus species (0.70%), Dermatophyte species (0.70%), Petrialla species (0.70%) and Trichophyton tonsurans (1.03%). Among the three different samples taken, ear swabs and fur swabs yielded the highest prevalence of fungal species (5.82% respectively) while nose swabs had the least prevalence of 3.43% of fungal infection.
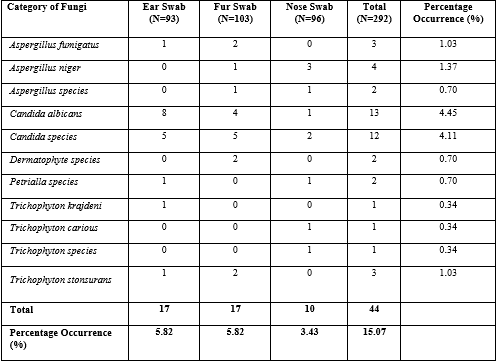
Table 4: Frequency and Percentage Occurrence of Fungal Isolates from Pet Dog Samples
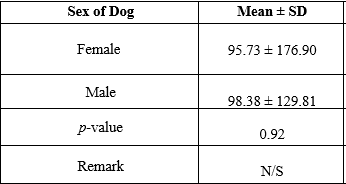
Table 5 compares the different organisms isolated and the type of sample, among the three swab samples taken, there was a significant difference in the isolation of microorganisms (P = 0.003) (p lessthan 0.05). The likelihood of isolating an organism from any of the three swab samples used in this research was significant (p = 0.00) (p lessthan 0.05). In contrast, table 6 compare the mean distribution of plate counts based on the sex or gender of the dogs. Female pet dogs have a mean ± SD of 95.73 ± 176.90 while the male counterparts had a value of 98 ± 129.81. The observed differences in the microbial plate count of the dogs based on gender were not statistically significant with a p-value of 0.92 (p lessthan 0.05).

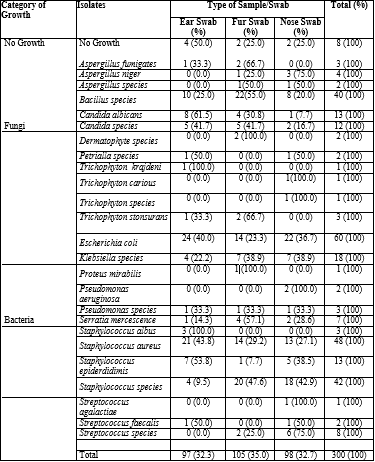
Table 4.5.: Chi-Square/Cross Tabulation of Isolates and Type of Sample/Swab
Pearson Chi-Square=81.521, df=50, N=300, p=0.003. Likelihood Ratio =93.067, df=50, N=300, p=0.00.
p < 0 Sig=Significant;> p > 0.05 = Not Significant=N/S
Table 6: t-test comparing Mean Distribution of Plate Counts based on Sex of Dogs
Table 7 compares the plate count for the different sample sites. The mean values for Ear Swab, Fur Swab and Nose Swab were 56.76 ± 79.43, 100.16 ± 138.43 and 136.04 ± 184.55 respectively. The observed differences in mean values of plate counts from the different sites were found to be statistically insignificant with a p-value of 0.31 (p lessthan 0.05)

P<0 Sig=Significant;>0.05 = Not Significant=N/S
Table 7: Mean Comparison (ANOVA) of Plate Count for Different Samples
Discussion
The ear swabs yielded the fewest positive cases (28.47%) compared to the fur and nose swabs of the pet dogs, which yielded 29.86% and 29.86%, respectively. Similarly, Abdel-moein et al., [13] reported that ear swab samples from the animals studied yielded the lowest number of positive cases. This observation may be attributable to the anatomical differences between the sampled sites, which may offer the animal in question more or less physical microbial protection. As shown in Table 2, the percentage distribution of bacteria on the dog's fur was 35.27% higher than on the other two sites, indicating a higher bacterial presence. As the outermost covering of the animal, the fur of pet dogs provides a relatively easier site for the deposition of bacterial species compared to the nose and ear. According to Hille et al., [14], Li et al., [15], and Eklund et al., [16], significant quantities of bacterial species have been isolated from the furs of pet dogs and other animals. These findings however are consistent with the above findings.
Out of 292 microorganisms isolated, this study revealed a cumulative prevalence of bacterial infection of 84.93% and fungal infection of 15.07%. This result was consistent with Talan et al., '[17] estimate of a 20%-83% infection rate, as well as Kettleson et al., [18] findings that a significantly greater number of bacterial species were isolated than fungal species. This observation could probably be attributed to the fact that bacterial species are the most prevalent microorganisms on the surface and in the cavities of dogs in both physiological and pathological states, followed by fungal species [19 ;20 ;21]. Nevertheless, Escherichia coli was the most prevalent organism in this study (20.55%), as shown in the result; the high E. coli prevalence recorded in this study is consistent with several studies where E. coli has been associated with the colonisation of the surfaces and fluids of pet and stray dogs [1], with the potential and capacity to transmit a further large amount of pathogenic organisms within the environment [22]; and resistant strains to humans as reported by Vega-Manriquez et al., [23]. However, Staphylococcus aureus was the second most prevalent organism isolated from this study, with a prevalence of 16.44%. This observation differs from the findings of Talan et al., [17], in which S. aureus was the most prevalent organism isolated in our study
Nonetheless, methicilin-resistant Staphylococcus aureus (MRSA) in apparently healthy and/or diseased pet dogs would make S. aureus an emerging veterinary pathogen that could probably potentially pose a threat to human health if it spreads to humans and within the eco-system [13]. However, the reason for these variations between the findings from this study and those of the cited authors is not known per se, nonetheless, it may probably be due to differences in study design and sample size. It may also be due to the method of analysis, skills and experience deployed at the time of laboratory analysis, even as the reason may be linked to the environmental and cultural behaviour of the people and also the location that hosted the study.
Bacillus species were also isolated in this study at a rate of 13.70% varying from the observations made by Azuonwu et al., [24] where Bacillus species were the least prevalent bacterial species. This variation could be attributed to the respective environment of the sampled pet dogs; the expertise in the bacteriological analysis may have also played a role. The genus Bacillus is well adapted to its environment; with a prominent species known as B. anthracis that causes Anthrax, a severe zoonotic disease that has a significant impact on human and animal health [25 ; 26]. Staphylococcus species were isolated from 14.38% of the studied animals which was similar to the observations made in a similar study by Azuonwu et al., [24]; these species are of public health significance because they are implicated in emerging zoonotic infections; a good example is Staphylococcus pseudintermedius, which colonises the skin and orifices of healthy canines and was recently identified as a cause of sinonasal infections in humans [27]. Furthermore, Klebsiella species (6.16%), which are potential causes of respiratory and urinary tract infections in humans, were also isolated from this study; though the prevalence in this study is in variance with a similarly designed study in Port Harcourt where this genus of bacteria was reported as 16%, though comparable with the findings of Marks et al., [28]. The ability of this genus to develop and spread extended-spectrum-lactamase (ESBL) enzymes in human populations is a major public health concern to be watched closely [29].
This study also led to the isolation of Staphylococcus epidermidis (4.4%), which are normal commensals of the skin and mucosa but are also opportunistic pathogens, with Meticillin-resistant (MR) and multidrug-resistant (MDR) isolates increasing in human, veterinary and healthcare settings[30], the study by Azuonwu et al. [24] however had a higher prevalence of other Staphylococcus species. Serratia marcescens was isolated from 2.39% of the pet dogs in this study; this organism is linked to severe and progressive cellulitis following a dog scratch [31]. The reported prevalence from this study was similar to the findings of Al-Kubaisi et al.,[32]. This study also led to the isolation of Streptococcus faecalis (0.60%), Streptococcus species (2.74%), Pseudomonas aeruginosa (0.68%), and Pseudomonas species (1.03%), some of which are capable of causing zoonotic infections in dog owners, as described by Damborg et al., [33] and have been observed in similarly designed studies with some level of variations [24; 28 ; 32]. Candida albicans was the most prevalent fungal isolate in this study, with a prevalence of 4.45%. This was closely followed by other Candida species, with a prevalence of 4.11%. This was not in agreement with Rees et al., [34], who asserted that the dermatophytic fungus, Trichophyton rubrum is the most common cause of skin infections in pet dogs. However, Nichita et al., [35] also recorded a higher prevalence of dermatophytes such as Microsporum canis and Trichophyton mentagrophytes compared to yeasts such as Candida albicans, which contradicts the results of the present study. Such primary infections have distinct morphologies and courses, are typically caused by a single organism, and typically affect healthy skin. However, Trichophyton species were the least prevalent in this study (Trichophyton Kragdeni, 0.34%; Trichophyton carious, 0.34%; and other Trichophyton species, 0.34%). Aspergillus fumigatus (1.03%), Aspergillus niger (1.37%), additional Aspergillus species (0.70%), Dermatophyte species (0.70%), Petrialla species (0.70%), and Trichophyton tonsurans (1.03%) were also isolated from this study. Although from this study, ear swabs and fur swabs yielded the highest prevalence of fungal species (5.82% and 5.82%, respectively), while nose swabs had the lowest prevalence of fungal infection (3.43%), however, in the study by Racine, [36], up to 20% of dogs have some form of ear disease and dogs are more susceptible to ear infections than humans due to the shape and structure of their ear [36; 34]. This study supported the conclusion of Racine [36] as it demonstrated a statistically significant difference in the isolation of microorganisms from ear swab samples (p=0.003) (p >0.05) with a statistically significant likelihood ratio (p= 0.00) (p lessthan 0.05).
Conclusion
In the present study, it was discovered that dogs harbour important organisms of public health significance in their nostrils, fur, and ears, with the likelihood of multidrug-resistant bacteria strains among these domestic dogs used as household pets. Additionally, the present study revealed a greater bacterial presence in the fur of the pet dogs than in the other two locations. Bacterial species were most predominant in the studied animals. In this study, Escherichia coli was the most prevalent bacterial species, followed by Staphylococcus aureus. Other bacterial species isolated in this study include Bacillus species, Staphylococcus species, Klebsiella species, Staphylococcus epidermidis, Serratia marcescens, Streptococcus faecalis, Streptococcus species, Pseudomonas aeruginosa, Pseudomonas species, the vast majority of which are capable of causing zoonotic diseases. Candida albicans were the most common Candida species isolated in this study, followed by other Candida species.
Recommendation
It is therefore, recommended that pet dogs should be vaccinated and properly treated when they are sick by trained personnel. Interestingly, much-needed personal hygiene must be observed at all times by the pet dog owners. Thus, kissing, caressing, eating and sleeping with dogs on the same bed must be avoided to reduce contact and cross-infection/contamination.
Conflicts of Interest
No conflict of interest among authors was reported.
Funding
No fund was received from any Organization.
Acknowledgement
We would like to thank the Veterinary Clinicians that helped very massively during the sample collections and also the Dog owners that allowed us to correct the sample from their pets after oral consent was granted by them. The laboratory staff of the University of Port Harcourt, Choba are not left out, as we are sincerely grateful to them all for their show of support and technical assistance during the laboratory analysis.
References
- Marchetti, L., Buldain, D., Gortari Castillo, L., Buchamer, A., Chirino‐Trejo, M., & Mestorino, N. (2021). Pet and Stray Dogs as Reservoirs of Antimicrobial-Resistant Escherichia coli. International Journal of Microbiology, 2021.
View at Publisher | View at Google Scholar - Da Costa, P. M., Loureiro, L., & Matos, A. J. (2013). Transfer of multidrug-resistant bacteria between intermingled ecological niches: the interface between humans, animals and the environment. International Journal of Environmental Research and Public Health, 10(1), 278-294.
View at Publisher | View at Google Scholar - Chomel B.B; Balotto, A & Meslin, F.X (2007). Widelife Exotic Pets and emerging zoonoses. Emerging Infectous Disease 13 (1), 6-13
View at Publisher | View at Google Scholar - Karesh, W. B., Dobson, A., Lloyd-Smith, J. O., Lubroth, J., Dixon, M. A., Bennett, M., & Heymann, D. L. (2012). Ecology of zoonoses: natural and unnatural histories. The Lancet, 380(9857), 1936-1945
View at Publisher | View at Google Scholar - WHO (2011). Managing zoonotic public health risks at the human animal-ecosystem interface. Geneva: Switzerland; WHO
View at Publisher | View at Google Scholar - Weese, J. S., & Kruth, S. A. (2006). Pets in voluntary household quarantine. Emerging Infectious Diseases, 12(6), 1029
View at Publisher | View at Google Scholar - Flynn, K. (1999). Overview of public health and urban agriculture: water, soil and crop contamination and emerging urban zoonoses. Cities feeding people series; rept. 30.
View at Publisher | View at Google Scholar - Schauss, K., Focks, A., Heuer, H., Kotzerke, A., Schmitt, H., Thiele-Bruhn, S., & Schloter, M. (2009). Analysis, fate and effects of the antibiotic sulfadiazine in soil ecosystems. TrAC Trends in Analytical Chemistry, 28(5), 612-618
View at Publisher | View at Google Scholar - Stull, J.W., Peregrine, A.S., and Sargeant, J.M. (2012). Household knowledge, attitudes and practices related to pet contact and associated zoonoes in Ontario, Canada. BMC Public Health; 12:553.
View at Publisher | View at Google Scholar - Stull, J. W., Brophy, J., Sargeant, J. M., Peregrine, A. S., Lawson, M. L., Ramphal, R., ... & Weese, J. S. (2014). Knowledge, attitudes, and practices related to pet contact by immunocompromised children with cancer and immunocompetent children with diabetes. The Journal of Pediatrics, 165(2), 348-355.
View at Publisher | View at Google Scholar - National Population Commission (NPC) (2006) Nigerian Population Census Repor. Accessed 12th September, 2022]
View at Publisher | View at Google Scholar - Cheesbrough M. District Laboratory practice in tropical countries. 2nd edition, Part Two Cambridge University Press. 2006;62-70
View at Publisher | View at Google Scholar - Abdel‐moein, K. A., El‐Hariri, M., & Samir, A. (2012). Methicillin‐Resistant Staphylococcus aureus: An Emerging Pathogen of Pets in Egypt with a Public Health Burden. Transboundary and emerging diseases, 59(4), 331-335.
View at Publisher | View at Google Scholar - Hille, K., Möbius, N., Akmatov, M. K., Verspohl, J., Rabold, D., Hartmann, M., ... & Kreienbrock, L. (2014). Zoonoses research in the German National Cohort. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz, 57(11), 1277-1282.
View at Publisher | View at Google Scholar - Li, Z., Liu, P., Cao, X., Lou, Z., Zaręba-Marchewka, K., Szymańska-Czerwińska, M., ... & Zhou, J. (2018). First report of Chlamydia abortus in farmed fur animals. BioMed research international, 2018.
View at Publisher | View at Google Scholar - Eklund, M., Aaltonen, K., Sironen, T., Raunio-Saarnisto, M., Grönthal, T., Nordgren, H., ... & Rantala, M. (2020). Comparison of Streptococcus halichoeri isolates from canine and fur animal infections: biochemical patterns, molecular characteristics and genetic relatedness. Acta Veterinaria Scandinavica, 62, 1-11.
View at Publisher | View at Google Scholar - Talan, D. A., Citron, D. M., Abrahamian, F. M., Moran, G. J., & Goldstein, E. J. (1999). Bacteriologic analysis of infected dog and cat bites. New England Journal of Medicine, 340(2), 85-92.
View at Publisher | View at Google Scholar - Kettleson, E. M., Adhikari, A., Vesper, S., Coombs, K., Indugula, R., & Reponen, T. (2015). Key determinants of the fungal and bacterial microbiomes in homes. Environmental Research, 138, 130-135.
View at Publisher | View at Google Scholar - Kasempimolporn, S., Saengseesom, W., Benjavongkulchai, M., & Sitprija, V. (2003). Oral Bacterial Flora of Dogs with and without Rabies: A Preliminary Study in Thailand. Journal of the Medical Association of Thailand, 86(12), 1162-1166
View at Publisher | View at Google Scholar - Verneuil, M., Durand, B., Marcon, C., & Guillot, J. (2014). Conjunctival and cutaneous fungal flora in clinically normal dogs in Southern France. Journal de Mycologie Medicale, 24(1), 25-28
View at Publisher | View at Google Scholar - Ma, G. C., Worthing, K. A., Ward, M. P., & Norris, J. M. (2020). Commensal Staphylococci Including Methicillin-Resistant Staphylococcus aureus from Dogs and Cats in Remote New South Wales, Australia. Microbial ecology, 79(1), 164-174
View at Publisher | View at Google Scholar - Anga, O. G., Monsi, T. P., Konne, F. E., & Mike-Ogburia, M. I. (2020). In Vitro Quantitative Assessment of Some Virulence Factors Produced by Escherichia coli in Different pH, Temperature and Oxygen Conditions. Advances in Microbiology, 10(12), 647-662.
View at Publisher | View at Google Scholar - Vega-Manriquez, X. D., Ubiarco-López, A., Verdugo-Rodríguez, A., Hernández-Chiñas, U., Navarro-Ocaña, A., Ahumada-Cota, R. E., & Eslava, C. A. (2020). Pet dogs potential transmitters of pathogenic Escherichia coli with resistance to antimicrobials. Archives of Microbiology, 1-7.
View at Publisher | View at Google Scholar - Azuonwu, O., Kemsi, B., Njoku-Tony, R. F., & Wokem, G. N. (2020). Investigation of Emerging Risk Factors and Isolation of Potential Pathogenic Bacteria from Domestic Dog Stool in Port Harcourt Metropolis, Niger Delta. Journal of Zoological Research, 1(2), 1-13.
View at Publisher | View at Google Scholar - Antwerpen, M., Pilo, P., Wattiau, P., Butaye, P., Frey, J., & Frangoulidis, D. (2012). Bacillus anthracis: Anthrax. In BSL3 and BSL4 Agents: Epidemiology, Microbiology, and Practical Guidelines, 1-18.
View at Publisher | View at Google Scholar - Okutani, A., Inoue, S., & Morikawa, S. (2019). Comparative genomics and phylogenetic analysis of Bacillus anthracis strains isolated from domestic animals in Japan. Infection, Genetics and Evolution, 71, 128-139.
View at Publisher | View at Google Scholar - Ference, E. H., Danielian, A., Kim, H. W., Yoo, F., Kuan, E. C., & Suh, J. D. (2019). Zoonotic Staphylococcus pseudintermedius sinonasal infections: risk factors and resistance patterns. International forum of allergy & rhinology, 9(7), 724-729.
View at Publisher | View at Google Scholar - Marks, S. L., Rankin, S. C., Byrne, B. A., & Weese, J. S. (2011). Enteropathogenic bacteria in dogs and cats: diagnosis, epidemiology, treatment, and control. Journal of Veterinary Internal Medicine, 25(6), 1195-1208.
View at Publisher | View at Google Scholar - Ewers, C., Bethe, A., Stamm, I., Grobbel, M., Kopp, P. A., Guerra, B., & Guenther, S. (2014). CTX-M-15-D-ST648 Escherichia coli from companion animals and horses: another pandemic clone combining multiresistance and extraintestinal virulence?. Journal of Antimicrobial Chemotherapy, 69(5), 1224-1230.
View at Publisher | View at Google Scholar - Schmidt, V. M., Williams, N. J., Pinchbeck, G., Corless, C. E., Shaw, S., McEwan, N., & Nuttall, T. (2014). Antimicrobial resistance and characterisation of staphylococci isolated from healthy Labrador retrievers in the United Kingdom. BMC veterinary research, 10(1), 1-14.
View at Publisher | View at Google Scholar - Pithadia, D. J., Weathers, E. N., Colombo, R. E., & Baer, S. L. (2019). Severe and progressive cellulitis caused by Serratia marcescens following a dog scratch. Journal of Investigative Medicine High Impact Case Reports, 7, 2324709619832330
View at Publisher | View at Google Scholar - Al-Kubaisi, S. M., Omar, A. S., Jassim, M. S., Mustafa, S. H., Maher, S. O., & Ahmed, S. J. (2020). Isolation and identification of facultative anaerobic bacteria from feces of pet dogs. Medico-legal update, 20(1), 785-789.
View at Publisher | View at Google Scholar - Damborg, P., Broens, E. M., Chomel, B. B., Guenther, S., Pasmans, F., Wagenaar, J. A., & Guardabassi, L. (2016). Bacterial zoonoses transmitted by household pets: state-of-the-art and future perspectives for targeted research and policy actions. Journal of Comparative Pathology, 155(1), 27-40.
View at Publisher | View at Google Scholar - Rees JR, Pinner RW, Hajjeh RA, Brandt ME, Reingold AL.(1998) The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: results of population-based laboratory active surveillanceexternal icon. Clin Infect Dis. 199827(5):1138-1147
View at Publisher | View at Google Scholar - Nichita Ileana and Mareu Adrian (2010) The fungal microbiota isolated from cats and dogs. Animal Science and Biotechnologies 43(1), 411-414.
View at Publisher | View at Google Scholar - Racine, E (2019) Dog Ear Infections: Symptoms, Causes, Treatment, and Prevention [Accessed 12 September, 2022].
View at Publisher | View at Google Scholar

 Clinic
Clinic
