Review Article | DOI: https://doi.org/10.31579/2835-785X/037
Coronavirus Disease 2019: Insight Into Genomic Structure, Life Cycle, Classification, Transmission, Prevalence, Clinical Impact, Laboratory Diagnosis, Its Effects on Hematological and Some Biochemical Parameters
1 Department of Physiology, Faculty of Medicine, Sabratha University, Libya.
2 Department of Biomedical Sciences, School of Basic Sciences, Libyan Academy, Tripoli, Libya.
*Corresponding Author: Azab Elsayed Azab, Department of Physiology, Faculty of Medicine, Sabratha University, Libya..
Citation: J M Jbireal, Azab E Azab, and Ruwaydah Ali Salem, (2024), Coronavirus Disease 2019: Insight Into Genomic Structure, Life Cycle, Classification, Transmission, Prevalence, Clinical Impact, Laboratory Diagnosis, Its Effects on Hematological and Some Biochemical Parameters, International Journal of Clinical Research and Reports. 3(1); DOI:10.31579/2835-785X/037
Copyright: © 2024, Azab Elsayed Azab. This is an open-access artic le distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 19 January 2024 | Accepted: 15 February 2024 | Published: 27 February 2024
Keywords: COVID-19; structure; life cycle; classification; prevalence; clinical impact; hematological and biochemical parameters
Abstract
Background: Coronaviruses can cause a variety of symptoms in infected humans ranging from mild cold-like symptoms to severe respiratory tract infections. It has become a global pandemic causing significant mortality and morbidity.
Objectives: The current review highlights genomic structure, life cycle, classification, transmission, prevalence, clinical impact, laboratory diagnosis, and its effects on hematological and some biochemical parameters. SARS-CoV-2 is a member of the betacoronavirus family within the Coronaviridae family and the subfamily Orthocoronavirinae. The coronavirus is a positive sense strand RNA virus, which is encapsulated by viral nucleocapsid (N) proteins to form a large ribonucleoprotein (RNP) complex. The coronavirus genome is composed of three main regions, including ORFs 1a and 1b. They are currently classified into four genera, including α- CoV, β-CoV, γ-CoV, and δ-CoV. It has been reported that the total number of COVID-19 deaths in Libya had reached 6433 patients by August 2022. The onset symptoms of COVID-19 infection are cough, breathing difficulties, fever, and fatigue which manifest after an incubation period lasting around 2 to 5 days. Other clinical symptoms are production of sputum, headache, diarrhea, dyspnea, and a reduction in lymphocyte count. The RT-PCR test is considered the gold standard for detecting SARS-CoV-2 and is the preferred laboratory diagnostic test for symptomatic patients in the acute phase. The serologic test is a type of enzyme-linked immunosorbent assay (ELISA) that detects the presence of SARS-CoV-2 antibodies (specifically IgM and IgG) in serum or plasma samples. Low thrombocytes, leukocytes and neutrophils counts were revealed in COVID -19 patient. Also, hemoglobin concentration is more affected by the COVID-19 infection than other RBC indices like MCH and MCHC. The coronavirus infection significantly increased the levels of serum urea, creatinine, fasting blood glucose, IgM, IgG, D-dimer, CRP, and ferritin at various times. Variations in renal function, electrolyte levels, and fasting blood glucose can all be useful markers of how a disease is progressing.
Conclusion: It can be concluded that This review shows that a genomic structure, life cycle, classification, transmission, prevalence, clinical impact, laboratory diagnosis, and its effects on hematological and some biochemical parameters.
Introduction
An aggressive virus known as the coronavirus disease 2019 (COVID-19) swept over the world and resulted in a pandemic [1]. The World Health Organization (WHO) proclaimed COVID-19 a global pandemic on March 11, 2020. Thus, the fight against COVID-19 is probably going to be a drawn-out marathon, and the pandemic has a significant effect on the health care systems of numerous nations [2]. COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has become a global pandemic causing significant mortality and morbidity and implementation of strict isolation measures [3]. It is a multisystemic disease that affects the immunological, hematopoietic, cardiovascular, gastrointestinal, and respiratory systems. The development of coronavirus disease 2019 signs and symptoms is influenced by white blood cells, hemoglobin, and platelets [1]. Serological testing can diagnose illness by detecting antibodies (IgM and IgG). Testing antibodies against SARS-CoV-2 is rapid and sensitive for the support diagnosis of COVID-19. The serum levels of CRP, D-dimers, and ferritin, which may be used in risk stratification to predict severe and fatal COVID-19 in hospitalized patients [3].
Objectives
The current review highlights genomic structure, life cycle, classification, transmission, prevalence, clinical impact, laboratory diagnosis, and its effects on hematological and some biochemical parameters.
Genome Structure
The COVID-19 virus is a tiny virus, with a diameter of approximately 65-125nm. It is a positive sense strand RNA virus, which is encapsulated by viral nucleocapsid (N) proteins to form a large ribonucleoprotein (RNP) complex. The virus also has an envelope membrane composed of lipids and viral proteins, including the spike (S), membrane (M), and envelope (E) proteins. Its genome is about 29.9 Kb in size and consists of at least ten Open Reading Frames (ORF) that encode 27 proteins. The genome is flanked by a 5' cap structure and a 3' poly-(A) tail [4].
The genome of SARS-CoV-2 is structured such that the 5' terminal two-thirds of the genome consists of ORF1a/b, which encodes two large polyproteins that form the viral repliase-transcriptase complex. Meanwhile, the remaining one-third of the genome contains the ORFs that encode the four structural proteins: S (spike), M (membrane), E (envelope), and N (nucleocapsid) proteins [5].
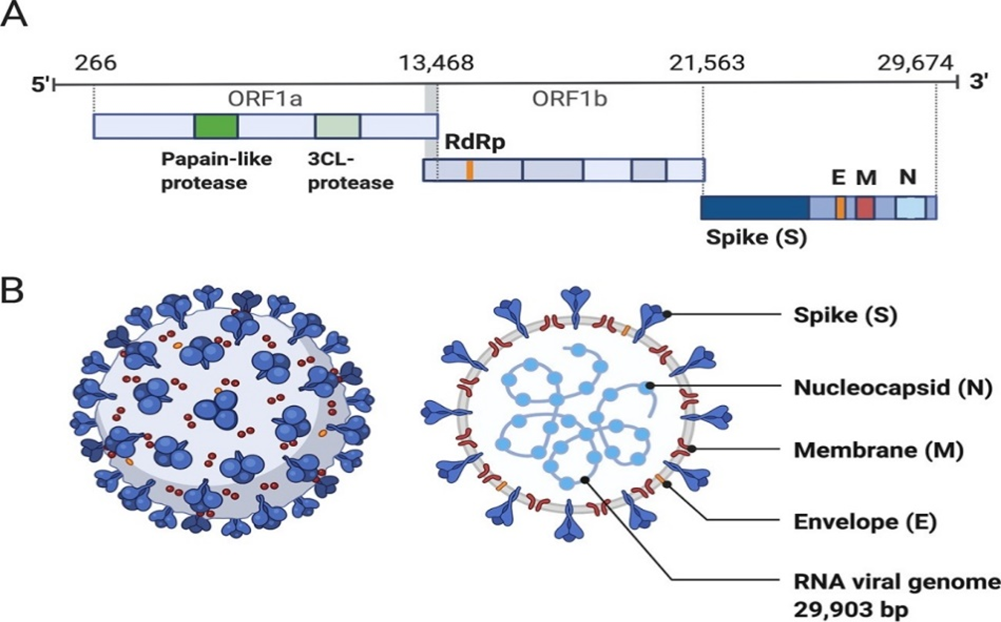
Figure 1: The structure of the SARS-CoV-2. capsid and genome.
The coronavirus genome is composed of three main regions, including ORFs 1a and 1b, which encode two polyproteins that generate nonstructural proteins (nsp), including important enzymes such as RNA-dependent RNA polymerase (RdRp). The final third of the genome encodes structural proteins, which include the spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins. Additionally, there may be accessory genes interspersed throughout the genome. The physical structure of the coronavirus virion is depicted in the figure, including the components that are determined by the conserved structural proteins S, E, M, and N [5].
COVID-19 Virus Replication Cycle:
The mechanism of SARS-CoV-2 entering the host cell involves the interaction between the virus's Spike (S) protein and specific receptors on the surface of target cells, namely the Angiotensin Converting Enzyme 2 receptors (ACE2). Once the virus binds to the receptor, it is internalized into the cell via endocytosis, allowing the viral genome to be released into the cytoplasm for replication. The host cell machinery then translates the viral genome to generate both structural and non-structural proteins [6].
The genome of the virus undergoes initial unpacking from N proteins, which are bound by cellular protease. Following this, the viral +g RNA functions directly as mRNA, translating the ORF1a and ORF1b regions, and as a template RNA for RNA transcription. The g RNA subsequently generates pp1a and pp1ab viral replicas polyproteins. The polyproteins synthesized from ORF1a and ORF1b are processed by two proteases, papain-like protease (PLpro) and 3C-like protease (3CLpro), encoded by ORFa-b, resulting in the release of sixteen non-structural proteins (NSPs). These NSPs are released both co-translationally and post-translationally from pp1a and pp1ab. These NSPs contain core enzymatic functions that are involved in RNA synthesis, RNA proofreading, and RNA modification [7]. However, it is important to note that all other viral structural and accessory proteins must be translated from newly synthesized Subgenomic RNA (Sg RNA). Ultimately, a newly generated g RNA is encapsulated with N proteins, enclosed by a viral envelope, and released from the infected cells [8].

Figure 2: Life cycle of SARS-CoV-2
The mechanism of SARS-CoV-2 entry into host cells involves binding of the S protein with ACE2 receptors present on the target cell surface, which can occur through endosomal (1a) or plasma membrane (1b) entry pathways, ultimately leading to the release of viral RNA into the host cell cytoplasm. The pp1a and pp1ab polyproteins are translated from this RNA, and cleaved by 3CLPRO and PIpro to form the RNA replicas transcription complex (RdRp) (2). The RdRp drives viral replication (3), leading to the production of structural proteins which form the spike, membrane and envelope of new virus particles (4). The proteins merge with the genomic RNA in the endoplasmic Reticulum-Golgi apparatus to form new virus particles, which are then transported out of the host cell via exocytosis [8].
Classification, Transmission, and Prevalence of Coronavirus
Coronaviruses have been known to cause viral respiratory infections worldwide since the 1960s, and are known to infect vertebrates, such as mammals, birds, snakes, and other wild animals [9, 10]. They are currently classified into four genera, including α- CoV, β-CoV, γ-CoV, and δ-CoV [11]. Among the common human coronaviruses are the alpha coronaviruses 229E and NL63, as well as the beta coronaviruses OC43 and HKU-1. Coronaviruses can cause a variety of symptoms in infected humans ranging from mild cold-like symptoms to severe respiratory tract infections [12]. Some human coronaviruses that have been transmitted from animals are associated with more severe symptoms such as Middle East Respiratory Syndrome (MERS), caused by the beta coronavirus MERS-CoV; severe acute respiratory syndrome (SARS), caused by the beta coronavirus SARS-CoV; and coronavirus disease 2019 (COVID-19), caused by the beta coronavirus SARS-CoV-2 [11].
SARS-CoV-2 is a member of the betacoronavirus family within the Coronaviridae family and the subfamily Orthocoronavirinae [13]. It is primarily transmitted through human-to-human contact via the inhalation or contact with infected droplets. Additionally, there is an evidence to suggest that the virus can also be transmitted via the fecal-oral route [14].
SARS-CoV-2 is an enveloped virus with a single-stranded positive-sense RNA genome that has infected numerous animal species and humans. Based on the large number of infected individuals who were exposed to the wet animal market in Wuhan, China, where live animals are routinely sold, it is suggested that this is the likely zoonotic origin of COVID-19. Recent investigations have raised the possibility of an alternative origin for the first transmission of SARS-CoV-2 to humans. These studies suggest that the virus may have been circulating undetected in human populations for an extended period, and only recently gained the ability to cause disease through genomic adaptations during human-to-human transmission [15, 16]. While coronaviruses typically remain confined to animal populations, they are believed to have undergone evolution over thousands of years, occasionally resulting in zoonotic outbreaks [17].
In January 2020, the virus responsible for COVID-19 was quickly isolated from infected patients and its genome was sequenced [18]. SARS-CoV-2 has since rapidly spread worldwide, largely through international travel. Initially, in December 2019, more than 90% of reported cases were in Hubei province, China. However, by March 2020, the highest prevalence of COVID-19 had shifted to Italy, the United States, Spain, France, Iran, and Germany [13].
During the week of 21 to 27 November 2022, the World Health Organization (WHO)reported that the number of new cases remained stable, increasing by 2% compared to the previous week. There were just under 2.7 million new cases reported (Figure .1). The number of new deaths decreased by 5%, with over 8400 new fatalities reported. As of 27 November 2022, there have been over 637 million confirmed cases and over 6.6 million fatalities reported globally [19].
Table 1: COVID-19 cases reported weekly by WHO Region, and global deaths, as of 27 November 2022 [19].
WHO Region | Cumulative cases (%) | Cumulative deaths (%) |
Western Pacific | 264 418 870 (15%) | 2 134 396 (4%) |
Europe | 181 861 099 (41%) | 282 552 (32%) |
Americas | 98 230 033 (29%) | 2 869470 (43%) |
South-East Asia | 60 642 718 (10%) | 801 676 (12%) |
Eastern Mediterranean | 23 191 725 (4%) | 348 896 (5%) |
Africa | 9 392 341 (1%) | 174 871 (3%) |
Global | 637 737 550 (100%) | 6 611 874 (100%) |
The initial COVID-19 case in Libya was reported on March 24, 2020, and approximately two months later, there was a notable increase in reported cases. The outbreak was initially concentrated in the southern region of Sabha and later spread to the eastern and western parts of the country. By August 2022, reports indicated that the total number of COVID-19 deaths in Libya had reached 6433 patients [16].
All districts in the West (except Aljfara) are in a very high incidence of community transmission (CT4) with Tripoli having the highest case incidence in the country. The testing rates in East and South are limited to assess to the level of community transmission. Twelve COVID-19 labs (out of 42) reported 2,357 (2,179 PCR) tests done in week 31. Thus, out of the (2.525.273) tests in Libya since the beginning of the response, 505,805 (20.0%) were confirmed positive for SARS-CoV-2 (COVID-19). The overall number of new cases reported in week 31 shows a 20
Clinical Impact
The onset of COVID-19 infection is marked by a range of symptoms such as cough, breathing difficulties, fever, and fatigue which manifest after an incubation period lasting around 2 to 5 days [20]. The duration of this period is influenced by the patient's age and immune system status. Other clinical symptoms of COVID -19 include the production of sputum, headache, diarrhea, dyspnea, and a reduction in lymphocyte count [21].
The COVID -19 disease is typically acute and self-limiting, with symptoms resolving after approximately two weeks from the time of infection. However, in some individuals, the infection can become more severe, leading to hospitalization and potentially referral to an Intensive Care Unit (ICU). In these cases, COVID -19 may progress to acute respiratory distress syndrome, septic shock [22], refractory metabolic acidosis, coagulation dysfunction, multiple organ failure, and ultimately death. The primary cause of death in COVID -19 cases is typically severe acute respiratory distress syndrome (ARDS), which is a consequence of direct viral damage to the lungs as well as an uncontrolled inflammatory response known as cytokine release syndrome. This syndrome is caused by the release of large amounts of pro-inflammatory cytokines and chemokinaes by immune cells reacting to cellular damage caused by the viral infection [23]. SARS-CoV-2 primarily infects the lower respiratory tract and alveoli, resulting in bilateral pneumonia with ground-glass opacity and patchy bilateral shadowing on computed tomography in approximately 50% of patients at the time of diagnosis [23, 24].
Laboratory Diagnosis
In the fight against COVID-19, rapid and precise detection has become a critical tool in preventing further spread. Contact tracing has also been identified as a vital measure. The RT-PCR test is considered the gold standard for detecting SARS-CoV-2 and is the preferred laboratory diagnostic test for symptomatic patients in the acute phase. To diagnosis by using real-time RT-PCR, RNA is extracted from respiratory tract samples such as nasopharyngeal or pharyngeal swabs, tracheal aspirate, sputum, and Broncho alveolar lavage [25, 26].
The PCR test for COVID-19 is specifically designed to detect whether an individual is currently infected with the virus. It is not capable of providing information about other diseases or symptoms [27], and it may not detect patients who have cleared the virus and recovered from the disease [28, 29]. Serology tests, on the other hand, are valuable in assessing the immune response [30], monitoring disease progression, and determining the duration of immune protection in individuals who have recovered from COVID-19 [31].
The serologic test is a type of enzyme-linked immunosorbent assay (ELISA) that detects the presence of SARS-CoV-2 antibodies (specifically IgG and IgM) in serum or plasma samples. The ELISA test utilized by the Centre of Disease Control (CDC) employs purified SARS-CoV-2 S protein, rather than live virus, as an antigen. Several test kits have been developed by various manufacturers that utilize serum, plasma, or whole blood samples, and can provide results in a matter of minutes with an accuracy of up to 95%. One such diagnostic rapid test kit, developed by Biologics (Singapore) and Abbott Laboratories (USA), has received emergency approval from the US Food and Drug Administration (FDA) and is currently the fastest SARS-CoV-2 detecting Point of Care Test (POCT) available, providing positive results in as little as 5 minutes and negative results in 13 minutes [32].
Effect of Covid-19 Infection on Haematological Parameters
According to Alnaas et al. [2], hemoglobin concentration is more affected by the COVID-19 infection than other RBC indices like MCH (pg/cell) and MCHC. The proportion of lymphocytes and the change in ferritin level had a weak negative connection (correlation coefficient (r) = -0.219 with P value of 0.001). During both infection episodes, the mean ferritin level increased dramatically (P-value = 0.000) to high levels. During the first week of infection, the percentage of anemia in males was somewhat lower than that in females (35.14% and 36.36%, respectively).
The identification of SARS-Cov-2 infection has become a major challenge in clinical practice, for social, financial and analytical reasons, the search in abnormalities in routine, low-cost and suggestive laboratory parameters are important to assist confirming in COVID- 19 diagnosis. The most Hematological findings including lymphocytopnia (decrease of peripheral blood lymphocyte count) According to a multicenter study including 1.099 patients from 552 site in China, lymphocytopnia was present in 83.2% of admitted patients. [33].
The studies which described by Terpos et al. [34] low lymphocyte count was presented in COVID -19 patient. Other study showed that lymphocyte decrease in the deceased population and rises in patient that recover [35]. Huang et al. [36], in a study in Wuhan, China, reported the epidemiological, clinical, laboratory and radiological characteristics, treatment and clinical results of 41 patients infected with SARS-CoV-2. In hospital admission, blood count showed leukopenia with leukocyte count below 4 × 109/L (25%) of 40 patients.
Thrombocytopenia also noted by Eren at al. [37] in this study they found that low thrombocytes, leukocytes and neutrophils counts were revealed in COVID -19 patient. In multicenter study by Guan at al. [33], thrombocytopenia (platelet count <150>
Effect of Covid-19 Infection on inflammatory factors and COVID-19-specific immunoglobulins
Elevated D-dimer levels have been frequently seen in patients with COVID- 19. Several meta-analyses have shown that D-dimer levels have prognostic value and correlate with disease severity and in-hospital mortality [38]. D-dimer can be an early marker to guide the management of COVID-19 patients [39]. Correlations of abnormal coagulation parameters with poor prognosis has been observed. Non-survivors have shown significantly higher levels of plasma D-dimer compared with survivors [40, 41]. Coagulopathy and overt disseminated intravascular coagulation appear to be associated with high mortality rates. Among the coagulation parameters, D-dimer elevation > 1 ug/L was the strongest independent predictor of mortality [41, 42].
Ferritin is an intracellular protein that contains iron and is the major form of iron stored in the cells. Although this protein is found in most tissue and organs; small amounts are secreted into the blood and are the carrier of iron. Ferritin is used as an indirect marker of the total amount of iron stored in the body. Ferritin concentration increases significantly during infection or cancerous conditions. Additionally, ferritin is an acute phase reactant that rises in the course of the disease [43].
The result of the study of Dahan et al. [44], who reported that a significant increase in ferritin levels was demonstrated in patients with moderate and severe disease, compared to patients with mild disease (P = 0.006 and 0.005, respectively). Severe patients had significantly higher levels of ferritin (2817.6 ng/ml) than non-severe patients (708.6 ng/ml) P = 0.02. Vakcanova [43], reported that patients with elevated ferritin levels (>200 ng/mL) had a higher incidence of severe illness when compared with those with normal ferritin levels (≤ 200 ng/mL) (50.0% vs 2.9%). In addition, the severity of illness manifested a significantly higher level of ferritin as compared with non-severe ones (median 921.3 vs 130.7 ng/mL, p < 0>
Also, Zhou et al. [41], found that ferritin levels were elevated in cases of COVID-19 with fatal outcomes compared with survivors. The CRP was elevated in 65% of COVID-19 patients on admission and elevated in 93.9% of severe COVID-19 patients (68). CRP levels are strong biological indicators to represent the severity of the COVID-19 infection. CRP seems to be one of the first biomarkers to show physiological complications in COVID- 19 patients
The diagnosis value of Antibodies test, according to the study has been done by Zhao, et al. [45], revealed a strong positive correlation between clinical severity and antibody titer since 2-week after illness onset, for the first time in COVID-19 patients. The results of this study suggested that a high titer of total antibodies against the virus may be considered as a risk factor of critical illness,
According to Azab et al. [3], as compared to the controls, coronavirus infection significantly increased the levels of IgM, IgG, D-dimer, CRP, and ferritin at various times. Additional research is required to validate these findings. Certain inflammatory factors and COVID-19-specific immunoglobulins in COVID-19 patients These variations in the levels of IgM, IgG, D-dimer, CRP, and ferritin during COVID-19 virus infection in COVID-19 patients may aid medical professionals in comprehending the COVID-19 and offering further clinical therapy alternatives.
Juanjuon [46], reported that IgM levels increased during the first week after COVID-19 infection, peaked for 2 weeks and then reduced to near-background levels in most patients. The immunoglobulin IgM antibody levels were slightly higher in deceased patients than recovered patients, but, in these groups, the immunoglobulin IgG antibody levels did not significantly differ. The researcher concluded that quantitative detection of IgM and IgG antibodies against COVID-19 quantitatively has potential significance for evaluating the severity and prognosis of COVID-19.
Hsueh et al. [47], reported that seroconversion for IgG (mean 10 days) occurred simultaneously, or 1 day earlier, then that for IgM and IgA (mean 11 days for both). IgG could be detected as early as 4 days after the onset of illness. The earliest time at which these three antibodies reached peak levels were similar (mean 15 days). A high IgG level (1:800) could persist for > 3 months. Long et al., [48] reported that Seroconversion for IgG and IgM occurred simultaneously or sequentially. After seroconversion, IgM and IgG titers were plateaued within 6 days. After 17-19 days of the onset of COVID- 19 symptoms, a positive virus-specific IgG was reached 100%, while after 20-22 days of the onset of COVID-19 symptoms, a positive virus- specific IgM reached a peak of 94.1%. Three weeks after the onset of the symptoms of COVID-19, the virus-specific IgM and IgG antibody titers were increased in patients [49].
Effect of Covid-19 Infection on blood glucose, renal function, and electrolytes
According to Azab et al. [50], coronavirus infections resulted in a drop in Na+ concentrations and increases in serum urea, creatinine, and fasting blood glucose. A noteworthy correlation was observed among many metrics. These biochemical alterations may offer more clinical therapy choices, aid in the prevention of the disease's major consequences, and improve doctors' understanding of COVID-19. As a result, clinicians should give COVID-19 patients' fasting blood glucose, renal function, and electrolyte state more consideration. Variations in renal function, electrolyte levels, and fasting blood glucose can all be useful markers of how a disease is progressing.
Prevention and Vaccination
Preventive measures to decrease the risk of COVID-19 infection include multiple strategies such as vaccination, adherence to social distancing guidelines, wearing masks in public, avoiding crowded areas, maintaining a safe distance from others, improving ventilation in enclosed spaces, managing potential exposure durations [51], frequent and thorough hand washing for at least twenty seconds, practicing good respiratory hygiene, and refraining from touching the face, especially the eyes, nose, and mouth with unwashed hands [52]. On December 2, 2020, the UK medicines regulator MHRA granted regulatory approval for the first COVID-19 vaccine [53]. The vaccine has also been evaluated for emergency use authorization (EUA) status by the US FDA and in several other countries [54]. However, initially, the US National Institutes of Health guidelines did not recommend any medication for the prevention of COVID-19 before or after exposure to the SARS-CoV-2 virus outside of a clinical trial setting.
Conclusion
It can be concluded that This review shows that a genomic structure, life cycle, classification, transmission, prevalence, clinical impact, laboratory diagnosis, and its effects on hematological and some biochemical parameters.
References
- References
View at Publisher | View at Google Scholar

 Clinic
Clinic
