Review Article | DOI: https://doi.org/10.31579/2835-2882/012
Clinical Pharmacology of Tobramycin
- Gian Maria Pacifici *
Professor of Pharmacology, via Sant’ Andrea 32, 56127 Pisa, Italy.
*Corresponding Author: Gian Maria Pacifici, Professor of Pharmacology, via Sant’ Andrea 32, 56127 Pisa, Italy.
Citation: Gian M. Pacifici, (2023), Clinical Pharmacology of Tobramycin, Clinical Research and Studies. 2(2); DOI:10.31579/2835-2882/012
Copyright: © 2023, Gian Maria Pacifici. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 14 March 2023 | Accepted: 24 March 2023 | Published: 03 April 2023
Keywords: breast-milk; cerebrospinal-fluid; efficacy-safety; meningitis; pharmacokinetics; placenta, prophylaxis; tobramycin; toxicity; treatment; trials
Abstract
Tobramycin is an important agent for treatment of many serious bacillary infections. The superior activity of tobramycin against Pseudomonas aeruginosa makes it the preferred aminoglycoside for treatment of serious infections caused by this organism. Tobramycin may be administered intravenously, intramuscularly, by inhalation, by ophthalmic application, or by a cream. The intramuscular or intravenous dose of tobramycin for treatment of gram-negative organisms is 5 to 7 mg/kg daily given to adult patients with normal renal function and the interval between doses should be expanded in patients with renal dysfunction. The efficacy and safety of tobramycin have been extensively reported but tobramycin may induce ototoxicity and/or nephrotoxicity in some patients but the toxicity is generally mild. The pharmacokinetics of tobramycin have been studied in patients with cystic fibrosis and in diseased patients and the elimination half-life of tobramycin is about 2 hours. The prophylaxis, treatment, and trials with tobramycin have been extensively reviewed. Tobramycin administered intravenously poorly penetrates into the cerebrospinal fluid but when tobramycin is administered intraventricularly or intrathecally along with intravenous administration treats the meningitis caused by Pseudomonas meningitis or by Acinetobacter meningitis. Tobramycin is poorly transferred across the human placenta and poorly migrates into the breast-milk. The aim of this study is to review tobramycin efficacy and safety, toxicity, pharmacokinetics, prophylaxis, treatment, trials, tobramycin penetration into the cerebrospinal fluid and treatment of bacterial meningitis, transfer of tobramycin across the human placenta, and tobramycin migration into the breast-milk.
Introduction
Tobramycin is an important agent for treatment of many serious gam-negative bacillary infections. Tobramycin may be given intramuscularly, intravenously, or by inhalation. Tobramycin also is available in ophthalmic ointment, solution, and cream. Tobramycin is absorbed slowly when it is applied topically in an ointment and somewhat more rapidly when it is applied as a cream. The superior activity of tobramycin against Pseudomonas aeruginosa makes it the preferred aminoglycoside for treatment of serious infections caused by this organism typically in combination with an antipseudomonal β-lactam antibiotic. The typical recommended intramuscular or intravenous dose of tobramycin used for the treatment of known or suspected gram-negative organisms as a single agent or in combination therapy for adults with normal renal function is 5 to 7 mg/kg daily. For patients with renal dysfunction the interval between doses should be expanded. For patients who are not candidates for expanded interval between doses a loading dose of 2 mg/kg and then 3 to 5 mg/kg per day given as divided doses over 8 to 12 hours is recommended. Dosages at the upper end of this range may be required to achieve therapeutic concentrations for trauma or burn patients, those with septic shock, patients with cystic fibrosis, and others in whom drug is more rapidly cleared and the volume of distribution is larger than the normal. Periodic determination of the plasma concentration of tobramycin is recommended strongly. Tobramycin may induce ototoxicity and/or nephrotoxicity but the toxicity is generally mild [1].
Tobramycin molecular structure (molecular weight = 467.515 grams/mole).
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “tobramycin efficacy and safety”, “tobramycin toxicity”, “tobramycin pharmacokinetics”, “tobramycin prophylaxis” “tobramycin treatment”, “tobramycin trials”, tobramycin CSF”, “tobramycin meningitis”, “tobramycin placental transfer”, and “tobramycin breast milk”. In addition, the book “The pharmacological basis of therapeutics” [1] has been consulted.
Results
Efficacy and safety of tobramycin
Treatment with tobramycin by inhaled solution or by inhaled powder effectively and safety decreased the density of Pseudomonas aeruginosa in sputum and reduced the hospitalization stay due to respiratory events [2]. The 4-week administration of a highly concentrated tobramycin solution for inhalation effectively improved pulmonary function and this treatment was safety and well-tolerated [3]. Tobramycin nebulized at 600 mg thrice-daily effectively improved clinical and pulmonary functions and reduced the density of Pseudomonas aeruginosa in sputum [4]. The short-term aerosolized administration of a high-dose of tobramycin in patients with cystic fibrosis is an efficacious and safe treatment for endobronchial infection caused by Pseudomonas aeruginosa [5]. Pseudomonas aeruginosa was effectively eradicated from the airways by inhaled tobramycin [6]. Long-term aerosolized tobramycin formulation in cystic fibrosis patients with chronic infection caused by Pseudomonas aeruginosa effectively improved pulmonary function and microbiologic outcome, decreased hospitalization stay, and this treatment was safe and well-tolerated [7]. In patients with bronchiectasis and chronic bronchial infection caused by Pseudomonas aeruginosa inhaled tobramycin effectively reduced the density of bacteria in sputum [8]. Tobramycin inhalation powder effectively reduced the density of Pseudomonas aeruginosa in sputum and produced improvements in pulmonary function [9]. Therapy with meropenem plus tobramycin or ceftazidime plus tobramycin effectively reduced the density of Pseudomonas aeruginosa in sputum [10].
Toxicity induced by tobramycin
Tobramycin was administered intravenously at a mean dose of 10.8 mg/kg daily to 4 patients who developed vestibule toxicity [11]. Tobramycin was inhaled to 10 patients with renal failure and tobramycin induced vestibule toxicity [12]. Tobramycin was administered intravenously to a patient and tobramycin induced bilateral high-frequency vestibular toxicity [13]. Nine or more doses of inhaled tobramycin were administered to 113 patients. Nephrotoxicity developed in 6.8% of patients and mild auditory toxicity developed in 13.1% of patients [14]. Tobramycin was administered intravenously to 118 immunocompromised patients with severe infections and nephrotoxicity and ototoxicity occurred in 17.1% and in 9.5% patients, respectively [15]. Tobramycin was nebulized at a dose of 300 mg twice-daily to a patient with pneumonia caused by Acinetobacter baumannii. The peak concentration ranged from 0.2 to 3.6 µg/ml and the trough concentration was undetectable making toxicity from this administration route negligible [16]. Forty-six patients with cystic fibrosis and chronic bronchopulmonary infection caused by Pseudomonas aeruginosa received tobramycin at a daily dose of 10 to 20 mg/kg. Eighteen patients (39.1%) had a reduced glomerular filtration-rate compared to normal values and 2 patients (4.3%) had a high-frequency hearing deficit. The toxicity caused by repeated high-dose of tobramycin in cystic fibrosis patients may be mild [17].
Pharmacokinetics of tobramycin in patients with cystic fibrosis
Whitehead et al. [18] studied the pharmacokinetics of tobramycin in 34 adult patients with cystic fibrosis who received a single intravenous dose of 10 mg/kg of tobramycin. Patients were aged 21 years (range, 16 to 32) weighted 55 kg (range, 40 to 73) and patients had Pseudomonas aeruginosa or Staphylococcus aureus in sputum.
Table 1. Pharmacokinetic parameters of tobramycin which have been obtained in 34 adult patients with cystic fibrosis. A single intravenous dose of 10 mg/kg of tobramycin was administered. Values are the mean+SD, by Whitehead et al. [18].
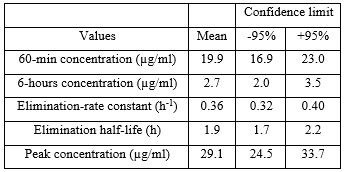
This table shows that tobramycin is rapidly eliminated as the mean elimination-rate constant and the mean elimination half-life are 0.36 h-1 and 1.9 hours, respectively, and there is a limited interindividual variability in pharmacokinetic parameters as the demographic characteristics of the patients included in the study range in a limited interval.
Schentag et al. [19] investigated the pharmacokinetics of tobramycin in 35 patients aged 63 years (range, 22 to 87). All patients had serious underlying heart or lung disease, diabetes, or complex postoperative problems. The site of infection varied 17 patients had pneumonia, 8 patients had pyelonephritis, 2 patients had bacterial endocarditis, and the reminder had abdominal or unidentified infections. Six patients (17.1%) had positive blood cultures, but none had septic shock, all patients had stable renal function, and tobramycin was intravenously infused. Table 2 provides the vital data of the patients included in the study and table 3 summarizes the pharmacokinetic parameters of tobramycin.
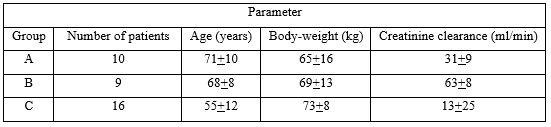
Table 2: Vital parameters of patients included in the study. Values are the mean+SD, by Schentag et al. [19].
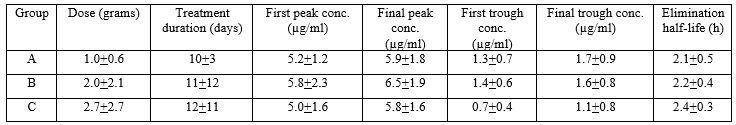
Table 3: Pharmacokinetic parameters of tobramycin which have been obtained in 35 diseased patients. Values are the mean+SD, by Schentag et al. [19].
This table shows that the mean first peak concentration is similar to the mean final peak concentration and the mean first trough concentration is similar to the mean final trough concentration indicating that tobramycin does not accumulate in serum. Tobramycin is rapidly eliminated as the mean elimination half-life is about 2 hours, the duration of treatment is similar in three groups of patients, and there is a remarkable interindividual variability in the pharmacokinetics parameters. This variability is accounted by a wide variation in patient vital data and disease.
Prophylaxis with tobramycin
The prophylaxis with piperacillin/tazobactam is similar to that with tobramycin in patients undergoing surgery [20]. Prophylaxis with tobramycin is superior to that with vancomycin in the prevention of infections caused by Staphylococcus aureus [21]. Preoperative tobramycin prophylaxis prior to colorectal surgery is associated with a significant decrease in surgical site infection and/or in the mortality-rate [22]. Prophylaxis with tobramycin effectively reduces the catheter-related infection in high-risk children on long-term haemodialysis [23]. Prophylaxis with tobramycin effectively reduces the occurrence of infections during the first week following renal transplantation [24]. Prophylaxis with topical tobramycin prevents the eye infection caused by Staphylococcus keratitis [25]. Prophylaxis with tobramycin is more efficacy than that with vancomycin in reducing the incidence of postoperative infections in patients undergoing cystectomy [26]. A single dose of 3.3 mg/kg of tobramycin effectively prevents the postoperative infection in patients undergoing elective colorectal surgery [27].
Treatment of bacterial infections with tobramycin
Tobramycin 0.3% eye-drops formulation provides an alternative therapy for acute bacterial conjunctivitis and improves patient compliance and satisfaction [28]. Tobramycin is more efficacious than gentamicin sulfate in the treatment of eye infection caused by Pseudomonas aeruginosa or by Staphylococcus aureus [29]. Piperacillin/tazobactam plus tobramycin is more effective and safer than ceftazidime plus tobramycin in treatment of patients with nosocomial lower respiratory-tract infection [30]. The once-daily high-dose regimen of tobramycin is safe and efficacious for treatment of patients with severe lower respiratory-tract infections [31]. Tobramycin effectively treats complicated urinary-tract infections caused by gram-negative organisms [32]. Inhaled tobramycin is well-tolerated and effectively treats patients with bronchiectasis caused by Pseudomonas aeruginosa and this treatment decreases the density of Pseudomonas aeruginosa in sputum and the hospitalization stay [33]. Treatment with inhaled tobramycin effectively cures the infection caused by Pseudomonas aeruginosa in sputum [34]. Treatment with inhaled tobramycin administered once-daily treats infection caused by Pseudomonas aeruginosa in sputum [35]. Inhaled tobramycin treats pulmonary infection caused by Pseudomonas aeruginosa in patients with cystic fibrosis [36]. Intermittent administration of inhaled tobramycin is well-tolerated, improves pulmonary function, decreases the density of Pseudomonas aeruginosa in sputum, and the hospitalization stay [37]. Tobramycin effectively treats lower respiratory-tract infection caused by sensitive bacteria in children [38].
Trials with tobramycin
Clinical trials with inhaled tobramycin showed that tobramycin effectively treats bronchiectasis in patients with bronchial infection [39]. Inhaled tobramycin effectively improves absolute change in forced expiratory volume and reduces the density of Pseudomonas aeruginosa in sputum indicating that tobramycin is an efficacious treatment [40]. Tobramycin inhalation powder has a safe and efficacy profile comparable to tobramycin inhalation solution and offers a more convenient treatment option for Pseudomonas aeruginosa lung infection in patients with cystic fibrosis [41]. Tobramycin nebulised solution significantly improves lung function of patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa [42]. Inhaled tobramycin reduces the density of Pseudomonas aeruginosa in sputum of patients with cystic fibrosis [43]. Both tobramycin sulphate 0.3% and moxifloxacin 0.5% eye drops are equally effective empirical treatments of acute bacterial conjunctivitis [44]. Tobramycin plus cefamandole is a safe and efficacious empiric therapy of infection caused by Pseudomonas aeruginosa in patients with cancer [45]. Clinical trials showed that tobramycin sulfate is a well-tolerated and is an effective treatment of infections caused by staphylococci in the respiratory-tract, central nervous system, skin, soft tissue, bone, and urinary-tract [46].
Penetration of tobramycin into the cerebrospinal fluid (CSF)
Concentration of tobramycin was measured in CSF of 11 patients undergoing neurosurgery and in 7 patients with meningitis following the intravenous administration of tobramycin at a dose of 80 mg daily. The mean tobramycin concentration in CSF was 0.47 µg/ml [47]. Tobramycin was administered at a dose of 5 to 10 mg into the lumbar intrathecal space and resulted in 27 to 81 µg/ml in the lumbar CSF, but only 0.0 to 2.1 µg/ml in the ventricular CSF. In contrast, tobramycin administered into the cerebral ventricle produced concentrations in lumbar CSF of 11.5 to 27.5 µg/ml and 12.8 to 40.0 µg/ml in the ventricular CSF [48]. Tobramycin was administered at conventional doses to 17 newborns and tobramycin concentration in the CSF is below 0.5 µg/ml [49]. Tobramycin was administered at conventional doses to 10 newborns and the CSF to serum ratio of tobramycin concentration is 0.14 [50]. These results indicate that tobramycin poorly penetrates into the CSF wend it is administered intravenously.
Treatment of bacterial meningitis with tobramycin
A child had the meningitis caused by Pseudomonas meningitis and was treated with tobramycin intraventricularly in conjugation with intravenous tobramycin and ceftazidime and the meningitis was cured [51]. An adult patient with meningitis caused by Acinetobacter meningitis was treated with tobramycin intrathecally and intravenously and this treatment successfully treated the meningitis [52]. An infant had the meningitis caused by Pseudomonas meningitis and tobramycin was administered intraventricularly at a dose of 2 mg and subsequent doses were adjusted to maintain a tobramycin trough concentration in the cerebrospinal fluid of 20 to 30 µg/ml and the meningitis was treated [53].
Transfer of tobramycin across the human placenta
Tobramycin was administered at a dose of 2 mg/kg to 35 pregnant women (13 first trimester, 22 second trimester) 0.5 to 34 h before hysterectomy and tobramycin concentration in the umbilical cord serum was lower than 0.6 µg/ml [54]. A single intramuscular dose of 40 mg of tobramycin was administered to 37 pregnant women before delivery and the mean peak level in the umbilical cord serum corresponded to 34.2% of that in the maternal serum [55]. A single dose of 2 mg/kg of tobramycin was given intravenously to a pregnant woman before delivery and the umbilical cord serum to maternal serum ratio was 0.35 [56]. These results indicate that tobramycin is poorly transferred across the human placenta.
Migration of tobramycin into the breast-milk
A single intramuscular dose of 80 mg of tobramycin was administered to 5 lactating women and milk levels were measured every hour for 6 hours. Only traces of tobramycin were detected in 4 of lactating women from 1 to 8 hours after the dose. In the fifth woman, milk levels ranged from 0.40 to 0.52 µg/ml over 8 hours with the highest level found at 4 hours after the dose [57]. A lactating woman received 80 mg of tobramycin every 8 hours by intramuscular injection. The milk concentration was 0.60 µg/ml 1 hour after the dose and 0.58 µg/ml 8 hours after the dose [58]. At 2 months postpartum, a lactating woman received tobramycin intravenously at a dose of 150 mg thrice-daily plus meropenem and the milk tobramycin concentrations were undetectable in 6 milk samples taken from 1 to 5 hours after the infusion [59]. These results indicate that tobramycin poorly migrates into the breast-milk.
Discussion
Tobramycin is an important agent for treatment of many serious gram-negative bacillary infections. The superior activity of tobramycin against Pseudomonas aeruginosa makes tobramycin the preferred aminoglycoside for treatment of serious infections caused by this organism typically in combination with an antipseudomonal β-lactam antibiotic. Tobramycin may be administered intravenously, intramuscularly, by inhalation, by ophthalmic ointment or solution, or by a cream. The dose of tobramycin to treat gram-negative organisms is 5 to 7 mg/kg daily in adult patients with normal renal function and in patients with renal dysfunction the interval between doses should be expanded [1]. The efficacy and safety of tobramycin have been extensively reported. Inhaled tobramycin effectively and safety decreases the density of Pseudomonas aeruginosa in sputum [2] and improved pulmonary function [3]. Nebulized tobramycin reduces the density of Pseudomonas aeruginosa in sputum [4]. Aerosolized tobramycin efficacy and safety treats endobronchial infection caused by Pseudomonas aeruginosa [5]. Inhaled tobramycin effectively eradicates Pseudomonas aeruginosa from the airways [6]. Long-term aerosolized tobramycin improves pulmonary function in cystic fibrosis patients with chronic infection caused by Pseudomonas aeruginosa, microbiologic outcome, and hospital stay [7]. Inhaled tobramycin effectively reduces the density of Pseudomonas aeruginosa in sputum [8, 9] and improves pulmonary function [9]. Meropenem plus tobramycin or ceftazidime plus tobramycin effectively reduces the density of Pseudomonas aeruginosa in sputum [10]. Tobramycin administered intravenously [11, 13, 15, 17] or inhaled [12, 14] or nebulized [16] causes ototoxicity and/or nephrotoxicity in some patients but the toxicity is generally mild. The pharmacokinetics of tobramycin have been studied by Whitehead et al. [18] in patients with cystic fibrosis and the mean elimination half-life of tobramycin is 1.9 hours. The pharmacokinetics of tobramycin have been studied by Schentag et al. [19] in patients with underlying heart or lung disease, diabetes, or complex postoperative problems but the renal function was stable in all patients and the mean elimination half-life of tobramycin ranges from 2.1 to 2.4 hours. The prophylaxis with tobramycin has been extensively reported. The prophylaxis with piperacillin/tazobactam is similar to that with tobramycin in patients undergoing surgery [20] and tobramycin is superior to vancomycin in preventing the infections caused by Staphylococcus aureus [21]. Prophylaxis with tobramycin decreases the surgical site infection and/or the mortality-rate in patients undergoing colorectal surgery [22], reduces the catheter-related infection in high-risk children on long-term haemodialysis [23], reduces the occurrence of infections in patients undergoing renal transplantation [24], and tobramycin is more effective than vancomycin in reducing postoperative infection in patients undergoing cystectomy [26]. Tobramycin prevents eye infection caused by Staphylococcus keratitis [25], and prevents postoperative infection in patients undergoing colorectal surgery [27]. The treatment of bacterial infections with tobramycin has been extensively reported. Tobramycin eye-drops treats bacterial conjunctivitis [28] and tobramycin is more effective than gentamicin sulfate in the treatment of eye infection caused by Pseudomonal aeruginosa or by Staphylococcus aureus [29]. Piperacillin/tazobactam plus tobramycin is more effective and safer than ceftazidime plus tobramycin in treatment of respiratory-tract infection [30]. Tobramycin effectively treats severe lower respiratory-tract infections [31] and complicated urinary-tract infections caused by gram-negative organisms [32]. Inhaled tobramycin treats bronchiectasis caused by Pseudomonas aeruginosa and decreases the concentration of this organism in sputum [33], the infection caused by Pseudomonas aeruginosa in sputum [34, 35], pulmonary infection caused by Pseudomonas aeruginosa in patients with cystic fibrosis [36], improves pulmonary infection, decreases the density of Pseudomonas in sputum, and the hospital-stay [37], and tobramycin treats respiratory-tract infection caused by sensitive organisms in children [38]. The trials with tobramycin have been extensively reported. Inhaled tobramycin effectively treats bronchiectasis in patients with bronchial infection [39], improves absolute change in forced expiratory volume and decreases the density of Pseudomonas aeruginosa in sputum [40], treats lung infection caused by Pseudomonas aeruginosa [41], and reduces the density of Pseudomonas aeruginosa in sputum [43]. Eye drops of both tobramycin sulfate and moxifloxacin equally treat bacterial conjunctivitis [44]. Tobramycin plus cefamandole effectively treat the infection caused by Pseudomonas aeruginosa in patients with cancer [45] and tobramycin sulfate treats the infections caused by staphylococci in the respiratory-tract, central nervous system, skin, soft tissue, bone, and in the urinary-tract [46]. The penetration of tobramycin into the cerebrospinal fluid has been reported. Tobramycin administered intravenously at a dose of 80 mg daily to patients undergoing neurosurgery and to patients with meningitis poorly penetrates into the cerebrospinal fluid [47]. Tobramycin administered at a dose of 5 to 10 mg into the lumbar intrathecal spaces resulted in significant concentrations in the lumbar cerebrospinal fluid and in poor concentrations in the ventricular cerebrospinal fluid. Tobramycin administered into the cerebral ventricle produced significant concentration in the ventricular cerebrospinal fluid [48]. Tobramycin administered at conventional dose reaches poor concentration in the cerebrospinal fluid of newborns [49, 50]. The treatment of bacterial meningitis with tobramycin has been reported. Tobramycin administered intraventricularly in conjugation with intravenous tobramycin and ceftazidime treats the meningitis caused by Pseudomonas meningitis in a child [51]. Tobramycin administered intrathecally and intravenously treats the meningitis caused by Acinetobacter meningitis in a patient [52]. Tobramycin administered intraventricularly produces a tobramycin trough concentration of 20 to 30 µg/ml and treats the meningitis caused by Pseudomonas meningitis in an infant [53]. Tobramycin is poorly transferred across the human placenta [54-56] and poorly migrates into the breast-milk [57-59].
In conclusion, tobramycin is an important agent for the treatment of many serious gram-negative bacillary infections. Tobramycin may be administered intramuscularly, intravenously, by inhalation, by ophthalmic ointment, solution, or by a cream. The efficacy and safety of tobramycin have been extensively reviewed but tobramycin may induce ototoxicity and/or nephrotoxicity in some patients and the toxicity is generally mild. The elimination half-life of tobramycin is about 2 hours, and the prophylaxis, treatment, and trials with tobramycin have been extensively reviewed. Tobramycin administered intravenously poorly penetrates into the cerebrospinal fluid but when tobramycin is administered into the cerebral ventricle produces significant concentrations in the cerebrospinal fluid. When tobramycin is administered intrathecally along with intravenous administration treats the meningitis caused by Pseudomonas meningitis or by Acinetobacter meningitis. Tobramycin is poorly transferred across the human placenta and poorly migrates into the breast-milk. The aim of this study is to review the clinical pharmacology of tobramycin.
Conflict of interests
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
References
- MacDougal C. (2018), Aminoglycosides. In The Goodman & Gilman’s. The Pharmacological Basis of the Therapeutics, Brunton Hilal-dandan LL, Knollmann BC, editors. Mc Graw Hill, 13th Edition, USA, New York; pp. 1039-1047.
View at Publisher | View at Google Scholar - Schwarz C, Taccetti G, Burgel P-R, Mulrennan S. (2022), Tobramycin safety and efficacy review article. Respir Med. 195: 106778.
View at Publisher | View at Google Scholar - Lenoir G, Antypkin YG, Miano A, Moretti P, Zanda M, et al. (2007), Efficacy, safety, and local pharmacokinetics of highly concentrated nebulized tobramycin in patients with cystic fibrosis colonized with Pseudomonas aeruginosa. Paediatr Drugs. 9(Suppl 1): 11-20.
View at Publisher | View at Google Scholar - Pai VB, Nahata MC. (2001), Efficacy and safety of aerosolized tobramycin in cystic fibrosis. Pediatr Pulmonol. 32(4): 314-327.
View at Publisher | View at Google Scholar - Ramsey BW, Dorkin HL, Eisenberg JD, Gibson RL, Harwood IR, et al. (1993), Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med. 328(24): 1740-1746.
View at Publisher | View at Google Scholar - Vázquez-Espinosa E, Girón RM, Gómez-Punter RM, García-Castillo E, Valenzuela C, Cisneros C, et al. (2015), Long-term safety and efficacy of tobramycin in the management of cystic fibrosis. Ther Clin Risk Manag. 11(3): 407-415.
View at Publisher | View at Google Scholar - Chuchalin A, Csiszér E, Gyurkovics K, Bartnicka MT, Sands D, et al. (2007), A formulation of aerosolized tobramycin (Bramitob) in the treatment of patients with cystic fibrosis and Pseudomonas aeruginosa infection: a double-blind, placebo-controlled, multicenter study. Paediatr Drugs. 9(Suppl 1): 21-31.
View at Publisher | View at Google Scholar - Elborn JS, Blasi F, Haworth CS, Ballmann M, Tiddens HWM, et al. (2022), Bronchiectasis and inhaled tobramycin: A literature review. Respir Med. 192: 106728.
View at Publisher | View at Google Scholar - Parkins MD, Elborn JS. (2011), Tobramycin Inhalation Powder™: a novel drug delivery system for treating chronic Pseudomonas aeruginosa infection in cystic fibrosis. Expert Rev Respir Med. 5(5): 609-622.
View at Publisher | View at Google Scholar - Blumer JL, Saiman L, Konstan MW, Melnick D. (2005), The efficacy and safety of meropenem and tobramycin vs ceftazidime and tobramycin in the treatment of acute pulmonary exacerbations in patients with cystic fibrosis. Chest. 128(4): 2336-2346.
View at Publisher | View at Google Scholar - Vonk SEM, Weersink EJM, Majoor CJ, Kemper EM. (2022), Tobramycin and vestibulotoxicity: retrospective analysis of four cases. Eur J Hosp Pharm. 29(1): e88-e90.
View at Publisher | View at Google Scholar - Edson RS, Brey RH, McDonald TJ, Terrell CL, McCarthy JT, et al. (2004), Vestibular toxicity due to inhaled tobramycin in a patient with renal insufficiency. Mayo Clin Proc. 79(9): 1185-1191.
View at Publisher | View at Google Scholar - Walsh RM, Bath P, Bance ML. (2000), Reversible tobramycin-induced bilateral high-frequency vestibular toxicity. ORL J Otorhinolaryngol Relat Spec. 62(3): 156-159.
View at Publisher | View at Google Scholar - Gatell JM, San Miguel JG, Zamora L, Araujo V, Bonet M, et al. (1983), Comparison of the nephrotoxicity and auditory toxicity of tobramycin and amikacin. Antimicrob Agents Chemother. 23(6): 897-901.
View at Publisher | View at Google Scholar - Bernstein JM, Gorse GJ, Linzmayer MI, Pegram PS, Levin RD, et al. (1986), Relative efficacy and toxicity of netilmicin and tobramycin in oncology patients. Arch Intern Med. 146(12): 2329-2334.
View at Publisher | View at Google Scholar - Kahler DA, Schowengerdt KO, Fricker FJ, Mansfield M, Visner GA, et al. (2003), Toxic serum trough concentrations after administration of nebulized tobramycin. Pharmacotherapy. 23(4): 543-545.
View at Publisher | View at Google Scholar - Pedersen SS, Jensen T, Osterhammel D, Osterhammel P. (1987), Cumulative and acute toxicity of repeated high-dose tobramycin treatment in cystic fibrosis. Antimicrob Agents Chemother. 31(4): 594-599.
View at Publisher | View at Google Scholar - Whitehead A, Conway SP, Etherington C, Caldwell NA, Setchfield N, et al. (2002), Once-daily tobramycin in the treatment of adult patients with cystic fibrosis. Eur Respir J. 19(2): 303-309.
View at Publisher | View at Google Scholar - Schentag JJ, Lasezkay G, Cumbo TJ, Plaut ME, Jusko WJ. (1978), Accumulation pharmacokinetics of tobramycin. Antimicrob Agents Chemother. 13(4): 649-656.
View at Publisher | View at Google Scholar - Shawar SK, Ly TV, Li J, Mary Shirk B, Reichert EM. (2020), Piperacillin/Tazobactam versus Tobramycin-Based Antibiotic Prophylaxis for Type III Open Fractures. Surg Infect (Larchmt). 21(1): 23-28.
View at Publisher | View at Google Scholar - Lee Y-P, Farhan S-D, Pendi A, Cunningham TJ, Kiester PD, et al. (2018), Does Addition of Tobramycin Powder Reduce Infection Rates After Spine Surgery? Global Spine J. 8(8): 816-820.
View at Publisher | View at Google Scholar - Mulder T, Crolla RMPH, Kluytmans-van den Bergh MFQ, van Mourik MSM, Romme J, et al. (2019), Preoperative Oral Antibiotic Prophylaxis Reduces Surgical Site Infections After Elective Colorectal Surgery: Results from a Before-After Study. Clin Infect Dis. 69(1): 93-99.
View at Publisher | View at Google Scholar - Onder AM, Chandar J, Billings A, Simon N, Gonzalez J, et al. (2009), Prophylaxis of catheter-related bacteremia using tissue plasminogen activator-tobramycin locks. Pediatr Nephrol. 24(11): 2233-2243.
View at Publisher | View at Google Scholar - Townsend TR, Rudolf LE, Westervelt FB Jr, Mandell GL, Wenzel RP. (1980), Prophylactic antibiotic therapy with cefamandole and tobramycin for patients undergoing renal transplantation. Infect Control. 1(2): 93-96.
View at Publisher | View at Google Scholar - Dajcs JJ, Moreau JM, Stroman DW, Schlech BA, Ke TL, et al. (2001), The effectiveness of tobramycin and Ocuflox in a prophylaxis model of Staphylococcus keratitis. Curr Eye Res. 23(1): 60-63.
View at Publisher | View at Google Scholar - Tanaka M, Matsumoto T, Ogata N, Masuda S, Kumazawa J. (1991), Preoperative oral and postoperative parenteral antibiotic prophylaxis of wound infection in total cystectomy with ileal urinary diversion. Urol Int. 47(1): 44-47.
View at Publisher | View at Google Scholar - Vanderveken M, Schepens M, Gerard Y. (1991), Prophylactic use of a single dose of tobramycin in elective colorectal surgery. Int Surg. 76(2): 127-130.
View at Publisher | View at Google Scholar - Kernt K, Martinez MA, Bertin D, Stroman D, Cupp G, et al. (2005), A clinical comparison of two formulations of tobramycin 0.3% eyedrops in the treatment of acute bacterial conjunctivitis. Eur J Ophthalmol. 15(5): 541-549.
View at Publisher | View at Google Scholar - Laibson P, Michaud R, Smolin G, Okumoto M, Rosenthal A, et al. (1981), A clinical comparison of tobramycin and gentamicin sulfate in the treatment of ocular infections. Am J Ophthalmol. 92(6): 836-841.
View at Publisher | View at Google Scholar - Joshi M, Bernstein J, Solomkin J, Wester BA, Kuye O. (1999), Piperacillin/tazobactam plus tobramycin versus ceftazidime plus tobramycin for the treatment of patients with nosocomial lower respiratory tract infection. Piperacillin/tazobactam Nosocomial Pneumonia Study Group. J Antimicrob Chemother. 43(3): 389-397.
View at Publisher | View at Google Scholar - Periti P. (1996), Tobramycin--clinical pharmacology and chemotherapy. J Chemother. 8(Suppl 1): 3-30.
View at Publisher | View at Google Scholar - Bailey RR, Peddie B. (1976), Tobramycin in the treatment of severe and complicated urinary tract infections. N Z Med J. 84(567): 1-3.
View at Publisher | View at Google Scholar - Tanriverdi E, Yildirim BZ, Gul S, Chousein EGU, Turan D, et al. (2021), Results of Tobramycin Inhalation Therapy in Patients with Noncystic Fibrosis Bronchiectasis with Pseudomonas aeruginosa Colonization: Real Life Management. J Aerosol Med Pulm Drug Deliv. 34(5): 274-279.
View at Publisher | View at Google Scholar - Nelson MT, Wolter DJ, Eng A, Weiss EJ, Brittnacher MJ, et al. (2020), Maintenance tobramycin primarily affects untargeted bacteria in the CF sputum microbiome. Thorax. 75(9): 780-790.
View at Publisher | View at Google Scholar - Mantero M, Gramegna A, Pizzamiglio G, D'Adda A, Tarsia P, et al. (2017), Once daily aerosolised tobramycin in adult patients with cystic fibrosis in the management of Pseudomonas aeruginosa chronic infection. Multidiscip Respir Med. 12:2.
View at Publisher | View at Google Scholar - Hamed K, Debonnett L. (2017), Tobramycin inhalation powder for the treatment of pulmonary Pseudomonas aeruginosa infection in patients with cystic fibrosis: a review based on clinical evidence. Ther Adv Respir Dis. 11(5): 193-209.
View at Publisher | View at Google Scholar - Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, et al. (1999), Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 340(1): 23-30.
View at Publisher | View at Google Scholar - Bothra M, Lodha R, Kabra SK. (2012), Tobramycin for the treatment of bacterial pneumonia in children. Expert Opin Pharmacother. 13(4): 565-571.
View at Publisher | View at Google Scholar - Vendrell M, Muñoz G, de Gracia J. (2015), Evidence of Inhaled Tobramycin in Non-Cystic Fibrosis Bronchiectasis. Open Respir Med J. 9(3): 30-36.
View at Publisher | View at Google Scholar - Galeva I, Konstan MW, Higgins M, Angyalosi G, Brockhaus F, et al. (2013), Tobramycin inhalation powder manufactured by improved process in cystic fibrosis: the randomized EDIT trial. Curr Med Res Opin. 29(8): 947-956.
View at Publisher | View at Google Scholar - Konstan MW, Flume PA, Kappler M, Chiron R, Higgins M, et al. (2011), Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. J Cyst Fibros. 10(1): 54-61.
View at Publisher | View at Google Scholar - Hodson ME, Gallagher CG, Govan JRW. (2002), A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur Respir J. 20(3): 658-664.
View at Publisher | View at Google Scholar - Nichols DP, Happoldt CL, Bratcher PE, Caceres SM, Chmiel JF, (2017), et al. Impact of azithromycin on the clinical and antimicrobial effectiveness of tobramycin in the treatment of cystic fibrosis. J Cyst Fibros. 16(3): 358-366.
View at Publisher | View at Google Scholar - Banerjee P, Chowdhury MSD, Ray S, Chatterjee S, Basak S, et al. (2020), Comparison of Tobramycin and Moxifloxacin eye drops in acute bacterial conjunctivitis: An open label randomized controlled institutional study from Kolkata. J Med Sci. 10(2): 110-114.
View at Publisher | View at Google Scholar - Feld R, Rachlis A, Tuffnell PG, Duncan I, Moran L, et al. (1984), Empiric therapy for infections in patients with granulocytopenia. Continuous v interrupted infusion of tobramycin plus cefamandole. Arch Intern Med. 144(5): 1005-1010.
View at Publisher | View at Google Scholar - Bendush CL, Weber R. (1076), Tobramycin sulfate: a summary of worldwide experience from clinical trials. J Infect Dis. 134(Suppl 1): S219-S234.
View at Publisher | View at Google Scholar - Brückner O, Alexander M, Collmann H. (1981), Tobramycin levels in cerebrospinal fluid of patients with slightly and severely impaired blood-cerebrospinal barrier. Chemotherapy. 27(5): 303-308.
View at Publisher | View at Google Scholar - Kaiser AB, McGee ZA. (1975), Aminoglycoside therapy of gram-negative bacillary meningitis. N Engl J Med. 293(24): 1215-1220.
View at Publisher | View at Google Scholar - Tessin I, Trollfors B, Thiringer K, Thörn Z, Larsson P. (1989), Concentrations of ceftazidime, tobramycin and ampicillin in the cerebrospinal fluid of newborn infants. Eur J Pediatr. 148(7): 679-681.
View at Publisher | View at Google Scholar - Farrington N, McEntee L, Johnson A, Unsworth J, Darlow C, et al. (2022), Pharmacodynamics of Meropenem and Tobramycin for Neonatal Meningoencephalitis: Novel Approaches to Facilitate the Development of New Agents to Address the Challenge of Antimicrobial Resistance. Antimicrob Agents Chemother. 66(4): e0218121.
View at Publisher | View at Google Scholar - Masvosva P, Buckingham SC, Einhaus S, Phelps SJ. (2003), Intraventricular and Intravenous Tobramycin with Ceftazidime for Ventriculitis Secondary to Pseudomonas aeruginosa. J Pediatr Pharmacol Ther. 8(2): 138-143.
View at Publisher | View at Google Scholar - Chicano-Piá PV, Cercós-Lletí A-C, Romá-Sánchez E. (2002), Pharmacokinetic model for tobramycin in acinetobacter meningitis. Ann Pharmacother. 36(1): 83-86.
View at Publisher | View at Google Scholar - Eiland LS, Luedtke SA, Chuachingco JC. (2004), Intraventricular tobramycin in a premature infant with pseudomonas meningitis. J Pediatr Pharmacol Ther. 9(1): 55-62.
View at Publisher | View at Google Scholar - Bernard B, Garcia-Cazares SJ, Ballard CA, Thrupp LD, Mathies AW, et al. (1977), Tobramycin: maternal-fetal pharmacology. Antimicrob Agents Chemother. 11(4): 688-694.
View at Publisher | View at Google Scholar - Yoshiokaab H, Mommaab T, Matsudaab S. (1972), Placental transfer of tobramycin. J Ped. (1): 121-123.
View at Publisher | View at Google Scholar - Fernandez H, Bourget P, Delouis C. (1990), Fetal levels of tobramycin following maternal administration. Obstet Gynecol. 76(5 Pt 2): 992-994.
View at Publisher | View at Google Scholar - Takase Z, Shirafuji H, Uchida M. (1975), Laboratory and clinical studies on tobramycin in the field of obstetrics and gynecology. Chemotherapy (Tokyo). 23:1399-1402.
View at Publisher | View at Google Scholar - Uwaydah M, Bibi S, Salman S. (1975), Therapeutic efficacy of tobramycin--a clinical and laboratory evaluation. J Antimicrob Chemother. 1(2): 429-437.
View at Publisher | View at Google Scholar - Festini F, Ciuti R, Taccetti G. (2006), Breast-feeding in a woman with cystic fibrosis undergoing antibiotic intravenous treatment. J Matern Fetal Neonatal Med. 19: 375-376.
View at Publisher | View at Google Scholar

 Clinic
Clinic
