Research article | DOI: https://doi.org/10.31579/2834-5126/018
Blood Circulation of the Rat Brain and Regulation Mechanisms
Grodno State Medical University, 80, Gorkogo St., 230009, Grodno, Republic of Belarus.
*Corresponding Author: Lizaveta I. Bon, Candidate of biological science, assistant professor of pathophysiology department named D.A. Maslakov, Grodno State Medical University, Belarus.
Citation: Bon E. I, Maksimovich N.Ye, Holik S.V, (2023), Blood Circulation of the Rat Brain and Regulation Mechanisms,J Clinical Trails and Clinical Research, 2(2); Doi: 10.31579/2834-5126/018
Copyright: © 2023, Bon E.I, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 04 April 2023 | Accepted: 12 April 2023 | Published: 24 April 2023
Keywords: bloodcirculation; bloodcirculation; regulation mechanisms
Abstract
In order to study circulatory disorders of the brain in rats and humans, adequate animal models are needed. The brain in humans and higher vertebrates, including rats, is intensively supplied with blood. The rat is one of the widely used objects of experimental research to study the pathology of cerebral circulation and its effect on the morphofunctional features of the cerebral cortex. For a possible extrapolation of the data obtained in the experiment to humans, it is necessary to have an idea of the peculiarities of the circulation of the brain in a rat.
Introduction
In order to study circulatory disorders of the brain in rats and humans, adequate animal models are needed. The brain in humans and higher vertebrates, including rats, is intensively supplied with blood. The rat is one of the widely used objects of experimental research to study the pathology of cerebral circulation and its effect on the morphofunctional features of the cerebral cortex. For a possible extrapolation of the data obtained in the experiment to humans, it is necessary to have an idea of the peculiarities of the circulation of the brain in a rat.
Vascular structures are an important element of the organization of the nervous system, which plays a decisive role in the functional activity and plasticity of neurons. Energy in the brain is produced by glucose oxidation, and therefore neuronal activity depends on the state of cerebral circulation and is extremely sensitive to its violation. Inadequate blood supply to the brain and a decrease in the functional activity of neurons is the cause of cerebrovascular pathology, leading to disability of the body, as well as death. [1]
Blood supply to the rat brain
According to modern concepts, the blood supply to the rat brain includes blood flow through two pairs of major blood vessels: internal carotid arteries and vertebral arteries. After the vertebral arteries reach the level above the cervical vertebrae, they merge into a common basal artery, which runs in a special hollow at the base of the bridge. The internal carotid artery gives off, among others, the anterior and middle cerebral arteries: the first branch branches in the corpus callosum and the inner surface of the hemisphere, the second branches on the outer surface of the hemisphere. [1,2].
The arteries immersed in the meninges and the vessels of the parenchyma of the brain have an endothelial layer, a well-developed basement membrane, a cytoskeleton, abundantly represented tile, lock, desmosomal, semi-desmosomal and dense contacts. More superficially lies a layer of circularly arranged smooth myocytes that form the media. Even more superficially is adventitia, which in the intracerebral arteries is a continuation of the subarachnoid space and forms the Virchow-Robbins space. The latter is gradually compressed and displaced by perivascular processes of gliocytes, primarily astrocytes.[3]
Macro- and microscopically it has been established that the arterial (willisian) circle in a rat resembles that of a human. The internal carotid arteries, having penetrated through a torn hole into the cranial cavity, are divided into caudal and nasal connective arteries; the latter at the level of the intersection of the optic nerves give the middle cerebral arteries. Having rounded the intersection of the optic nerves, the nasal connective arteries plunge into the longitudinal cleft of the brain, where they merge into an unpaired nasal cerebral artery or follow in parallel with separate trunks. In front of the intersection of the optic nerves, both nasal connective arteries are often connected to each other by a thin postchiasmatic branch, similar to the anterior connective artery in humans. The caudal connective arteries merge with the caudal cerebral arteries, which are branches of the main artery (Picture 1).
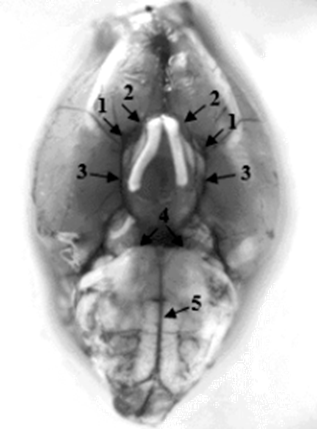
Picture 1: The structure of the Willisian circle in a white rat. 1 – internal carotid artery; 2 – nasal connective artery; 3 – caudal connective artery; 4 – caudal cerebral artery; 5 – the main artery.
In 75% of cases, the rat has a closed willisian circle, in which the nasal connective arteries in front of the intersection of the optic nerves are connected by a thin postchiasmatic branch (Picture 1). In 25% of cases, there is an open Willis circle, in which the right and left nasal connective arteries do not anastomose with each other, but each pass into the corresponding nasal cerebral artery. In 50% of the observations, the arterial circle of the white rat has the shape of an "eight" (Picture 2). In this case, the caudal connective arteries near the bridge are connected by a thin arterial vessel (an additional connective artery), which divides the Willis circle into two rings of different diameters (the larger one is cranial and the smaller one is caudal).
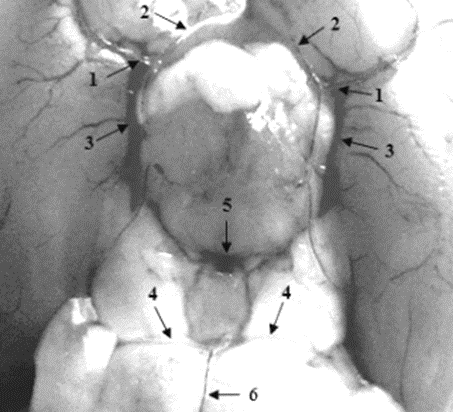
Picture 2. The Willis circle of the white rat in the form of an "eight".
1 – internal carotid artery; 2 – nasal connective artery; 3 – caudal connective artery; 4 – caudal cerebral artery; 5 – the artery connecting the caudal connective arteries; 6 – the main artery
Analysis of the morphometric parameters of the vessels showed that the Willis circle in the rat has a variant of the structure of the posterior trifurcation of the internal carotid on both sides (large caudal connective and caudal cerebral arteries). Due to the larger caliber of the internal carotid arteries compared to the main artery, the main blood flow (90%) to the brain is carried out through the internal carotid arteries.[1-3]
The Willis circle in rats is identical in anatomy and sources of formation (internal carotid and main arteries) to the arterial circle of the human large brain. The vessels of the arterial circle of the brain of these animals are similar to the arteries of the Willisian circle in humans: the nasal connective arteries are similar to the anterior cerebral arteries of humans, the postchiasmatic branch corresponds to the anterior connective artery, the caudal connective arteries correspond to the posterior connective arteries, and the caudal cerebral arteries correspond to the posterior cerebral arteries. In rats, the postchiasmatic artery is an anastomosis between the nasal connective arteries and unites both internal carotid arteries. The caudal connective arteries are an important anastomosis between the carotid and vertebral artery systems. [4]
The greatest differences in the structure of the Willis circle in rats are manifested in the variation in the presence of a postchiasmatic artery, as well as in the difference in their morphometric characteristics and bilateral dissymmetry of anatomical structure and quantitative indicators.
Venous branches, formed from several capillaries, flow into the trunk of the intracerebral vein at a right or an obtuse angle. The large veins of the brain surface are constructed from two layers of the endothelium: the endothelium of the vein itself and the endothelium of its vagina with a layer of amorphous substance located between them. Adventitia of veins appears only in the case of their fibrosis.
The volume of the venous vessels of the brain is 2-3 times the volume of the arterial system. The regulating role of the venous system primarily consists in the redistribution of pressure in the vascular system of the brain. In the cavernous sinuses, the evacuation of blood is facilitated by pulsating segments of the main arteries. The reverse outflow of blood through the veins into the skull is difficult due to the presence of valves in them that prevent blood circulation. The pressure in the venous sinuses of the brain is not the same, which is due to their anatomical features.[5]
The connecting link between the arteries and veins of the brain is a network of capillary vessels. The more functionally significant the structures of the brain are, the more intensive their metabolism is and the richer the angioarchitectonics of capillaries is represented.
The blood capillaries of the rat brain have a number of common organizational features that bring them closer to similar microvessels in organs with pronounced barrier properties. At the same time, they are distinguished by the absence of a connective tissue environment. Two types of cells are involved in the formation of blood capillaries – endotheliocytes and pericytes. Endotheliocytes are polarized cells with apical and basal surfaces. Morphologically, the endothelium of the capillaries of the brain has no fenestra, surrounded by pericytes and a well-defined and continuous basement membrane. Differentiated endotheliocytes are characterized by an insignificant content of membrane organelles, with the exception of mitochondria and a small number of vesicles. There are a large number of desmosome-like compounds between the cells. In places where there is no hemato-encephalic barrier (neuroendocrine nuclei of the hypothalamus, some areas of the brain parenchyma in the immediate environment of the III ventricle and around the cavity of the IV ventricle), blood capillaries have a thinned, fenestrated endothelial lining, which has a high degree of permeability to macromolecular compounds, hormones. In these zones, a well-developed system of small pores is revealed, and therefore the barrier properties are poorly expressed.[6-8]
Pericytes are grouped around the endothelium of capillaries and are contractile cells that change the diameter of the lumen and provide adapted blood flow. In large vessels, this role belongs to smooth myocytes. Pericytes contain many actin-like microfilaments. Astrocytes form a dense pericapillary case.
The study of changes in the blood vessels of the cerebral cortex, corpus callosum, septum and caudate nucleus of the rat brain using angiography and histological analysis revealed the uniformity of the distribution of vessels in each of the studied areas. However, the density of vessels per unit volume in different parts of the brain is different. The distribution of microvessels in the parenchyma of the brain is the most important indicator of energy consumption and trophic provision of neurons, depending on the removal of microvessels from the neuron, the size and shape of the cell body, hemodynamic conditions, the content of oxygen and nutrients in the flowing blood.[6]
Connection of angioarchitectonics with cytoarchitectonics of the brain
The presence of a modular organization of neurons in the cerebral cortex is associated with a similar structure of vascular networks, since the formation of the latter depends on the organization of neural ensembles. However, vascular modules and their relationship with the cytoarchitectonics of the brain have not been studied enough. In cortical models of the primary visual and somatosensory cortex, the vascular organization corresponds to neuronal cytoarchitectonics. Vascular structures correspond to the boundaries of neural modules and are surrounded by astrocytes that isolate the structural and functional units of the brain.
In general, the cerebral vascular architecture, which determines cerebral blood flow and oxygen metabolism in the brain, has not been sufficiently studied to date. There are only isolated studies in this area that indicate the connection of angioarchitectonics with cytoarchitectonics of the brain in rats and humans. Thus, in the somatic sensory cortex, the differential distribution of microvessels between cortical modules is consistent with the distribution of mitochondrial enzymes (for example, cytochrome oxidase) and with electrical and metabolic neuronal activity. Cytochrome oxidase activity of mitochondria and microvessel density correlate in the rat parietal cortex, which is confirmed by studies on the analysis of succinate dehydrogenase activity. At the same time, the distribution of blood vessels is dynamic and depends on the activity of brain departments.[9,10]
During the study of brain reactions to damage, as well as studies of age-related changes in the cytoarchitectonics of the rat brain, it was found that in the somatosensory cortex and nuclear centers of the trunk, the distribution of microvessels in the experiment is somewhat different from changes in its neuroarchitectonics and the energy activity of the bodies of neurons and neuropiles. However, with age, significant structural rearrangements of the capillary network are noted, which is accompanied by structural and functional transformations of astrocytes. The dynamics of the astrocytic-vascular environment in the age aspect is most pronounced precisely in the long term after damage and structural and functional rearrangements of the brain and is largely irreversible.
The connection between vessels and neurons is also due to the level of endothelial permeability, the functional activity of neurons by neurotransmitter-hormonal factors (norepinephrine, serotonin, acetylcholine and GABA).
Regulation of cerebral circulation
The mechanisms of regulation of blood circulation are conditionally divided into central (neurohumoral) and local (peripheral, or regional).
The blood circulation of the brain is more intense than that of other organs. The brain needs a constant supply of O2 and nutrients, blood flow to the brain is relatively independent of the minute volume of blood and the activity of the autonomic nervous system. CNS cells with insufficient oxygen supply cease to function earlier than cells of other organs. The cessation of blood flow to the cat's brain for 20 seconds causes the complete disappearance of electrical processes in the cerebral cortex, and the cessation of blood flow for 5 minutes leads to irreversible damage to neurons.[8]
Systemic mechanisms of blood circulation regulation
Systemic mechanisms of blood circulation regulation are presented in the diagram and include nervous and humoral regulate.
Nervous regulation
About 15% of the blood from the cardiac output enters the vessels of the brain. Nervous regulation of blood flow is provided by a complex mechanism, including sensitive, central and efferent links.[4]
Cerebral arteries are muscle-type vessels with abundant adrenergic innervation, which allows them to change the lumen within a wide range.
The sensitive link. Vascular receptors – angioreceptors – by their function are divided into baroreceptors (pressoreceptors) that respond to changes in blood pressure (BP), and chemoreceptors that are sensitive to changes in the chemical composition of blood. The largest concentrations of them are in the main reflexogenic zones: aortic, sinocarotid, vessels of the pulmonary circulation. The irritant of the baroreceptors of the aortic arch, carotid sinus, intra-thoracic and cervical arteries is the rate and degree of stretching of the vessel wall by pulse or increasing fluctuations in blood pressure. Baroreceptor reflexogenic zones can be pressor and depressor. Thus, when the pressure drops, the intensity of the pulse from the baroreceptors decreases, which leads to a reflex increase in the tone of the muscle cells of the vascular wall due to the activation of the pressor zone of the vasomotor center. Accordingly, the total peripheral vascular resistance increases, leading to normalization of blood pressure. Impulses coming from the depressor zones of the vasomotor center have the opposite effect.
Chemoreceptors of carotid bodies in the area of bifurcation of the common carotid arteries and the aortic arch react to changes in the concentration of O2, CO2, H+, some inorganic and organic substances in the blood. Hypoxia, hypercapnia, accompanied by a change in the chemical composition of the blood, lead to the emergence of cardiovascular and respiratory reflexes, which are aimed at normalizing the gas composition of the blood and maintaining homeostasis. Reflexes arising from the receptor zones of the cardiovascular system and determining regulation within its limits are called proper (systemic) circulatory reflexes. With an increase in irritation, in addition to the cardiovascular system, respiration is involved in the response - a conjugate reflex. The thresholds of irritation for own reflexes are lower than for conjugated ones. The existence of conjugated reflexes enables the circulatory system to adapt quickly and adequately to the changing conditions of the internal environment of the body.[11]
Carotid chemoreceptors are more involved in changes in pulmonary ventilation, aortic chemoreceptors are mainly involved in changes in the activity of the cardiovascular system. Chemoreceptors are also found in the vessels of the heart, spleen, kidneys, bone marrow, digestive organs, etc. Their physiological role consists in the perception of osmotic blood pressure and the transmission of a signal about its change in the central nervous system.
The mechano- and chemoreceptors of the vessels are also located in the walls of the vessels of the venous bed. Thus, an increase in pressure in the veins of the abdominal cavity is accompanied by a reflex increase and deepening of breathing, the suction action of the chest and an increase in cardiac blood flow.
The central link nervous regulation is represented by the vasomotor (vasomotor) center. Structures related to the vasomotor center are localized in the spinal cord, medulla oblongata, hypothalamus and cortex of the cerebral hemispheres.
• Spinal regulation level. Nerve cells whose axons form vasoconstrictive fibers are located in the lateral horns of the thoracic and first lumbar segments of the spinal cord.
• Bulbar level of regulation. The vasomotor center of the medulla oblongata is the main center for maintaining vascular tone and reflex regulation of blood pressure.
The vasomotor center contains depressor, pressor and cardioinhibiting zones. This division is conditional due to their overlap.
The activity of the depressor zone helps to reduce blood pressure by reducing the activity of sympathetic vasoconstrictor fibers, thereby causing vasodilation and a decrease in total peripheral resistance, as well as by weakening sympathetic stimulation of the heart and reducing cardiac output. The depressor zone is the switching point of impulses coming from the baroreceptors of reflexogenic zones, which cause central inhibition of tonic discharges of vasoconstrictors. Also, the depressor area suppresses the pressor zone and increases the activity of the parasympathetic system.
The pressor zone has the opposite effect, when it is activated, an increase in blood pressure occurs due to an increase in the total peripheral vascular resistance and cardiac output. The interaction of depressor and pressor structures of the vasomotor center has a complex synergistic-antagonistic character.
The effects of the third zone (cardioinhibitory) are mediated by the fibers of the vagus nerve going to the heart. An increase in its activity leads to a decrease in cardiac output, complementing the effect of the depressor zone in lowering blood pressure.[7]
The state of tonic excitation of the vasomotor center and, accordingly, the blood pressure level are regulated by impulses coming from vascular reflexogenic zones. In addition, this center is part of the reticular formation of the medulla oblongata, from where it also receives numerous collateral excitations.
The influence of the vasomotor center is carried out through the spinal cord, the nuclei of cranial nerves (VII, IX and X pairs), peripheral formations of the autonomic nervous system.
The vasomotor center of the medulla oblongata interacts with the hypothalamus, cerebellum, basal nuclei, and cerebral cortex in reactions at the body level. It mediates urgent responses of the vascular system during intense muscle work, hypoxia, hypercapnia, acidosis.
• The hypothalamic level of regulation also plays an important role in the implementation of adaptive circulatory reactions. The integrative centers of the hypothalamus exert a downward influence on the cardiovascular center of the medulla oblongata, providing differentiated phase and tonic control. In the hypothalamus, as in the bulbar vasomotor center, there are depressor and pressor zones. This allows us to consider the hypothalamic level of regulation as a superstructure acting as a kind of understudy of the bulbar center.
• The cortical level of regulation has been studied in detail using the methods of conditioned reflexes. It is relatively easy to develop a vascular reaction to a previously indifferent stimulus, while causing a feeling of heat, cold, pain, etc.
Certain areas of the cerebral cortex, like the hypothalamus, have a downward influence on the main center of the medulla oblongata. These influences are formed as a result of comparing the information that has entered the higher department of the nervous system from various receptor zones with the previous experience of the body. They provide the implementation of the cardiovascular component of emotions, motivations, behavioral reactions.
Efferent link. Efferent regulation of blood circulation is realized through nervous and humoral mechanisms.
The nervous mechanism is carried out with the participation of 3 components.
1 preganglionic sympathetic neurons, whose bodies are located in the anterior horns of the thoracic and lumbar spinal cord, as well as postganglionic neurons lying in the sympathetic ganglia.
2 preganglionic parasympathetic neurons of the nucleus of the vagus nerve located in the medulla oblongata, and the nucleus of the pelvic nerve located in the sacral spinal cord, and their postganglionic neurons.
3 for hollow visceral organs – efferent neurons of the metasympathetic nervous system, localized in the intramural ganglia of their walls. They represent the common final path of all efferent and afferent influences that act on the heart and blood vessels through adrenergic, cholinergic and other regulatory links.
All vessels are subject to innervation, with the exception of capillaries. The innervation of the veins corresponds to the innervation of the arteries, although its density is much less. The nerve endings of the efferent fibers are precisely traced to the precapillary sphincters, where they end at smooth muscle cells. Sphincters are able to actively respond to incoming impulses.[3,8]
The main mechanism of nervous regulation is efferent innervation of a non-synaptic type by means of free diffusion of mediators in the direction of the vessel wall.
The effect of adrenaline and norepinephrine on the vascular wall is determined by the existence of different types of adrenoreceptors – α and β, which are areas of smooth muscle cells with special chemical sensitivity. Vessels usually have both types of receptors. The interaction of the mediator with the α-adrenoreceptor leads to a contraction of the vessel wall, with the β-receptor – to relaxation.
α1- and β1-receptors are localized mainly on postsynaptic membranes and react to the action of norepinephrine released from the nerve endings of postganglionic neurons of the sympathetic department.
α1 receptors are localized in arterioles, their stimulation leads to spasm of arterioles, increased pressure, decreased vascular permeability.
β1 receptors are localized in the heart, their stimulation leads to an increase in frequency (positive chronotropic effect) and the strength of heart contractions (positive inotropic effect), contributing to an increase in blood pressure, increases the myocardial oxygen demand. Also, β1 receptors are localized in the kidneys, being receptors of the juxtaglomerular apparatus.
α2- and β2-receptors are extra-synaptic, and are also present on the presynaptic membrane of the same neurons.
α2 receptors are localized mainly at the presynaptic terminal. Norepinephrine acts on the α2 receptors of the presynaptic membrane according to the principle of negative feedback – it inhibits its own release (the "negative feedback loop" of the adrenergic system), their stimulation leads to a decrease in blood pressure. Both epinephrine and norepinephrine act on α2 receptors.
β2 receptors are sensitive mainly to adrenaline. When adrenaline acts on the β2-adrenoreceptors of the presynaptic membrane, the release of norepinephrine increases. These receptors are localized in bronchioles and on liver cells.
Serotonin and dopamine are vasoconstrictors. The mediator serotonin through a number of receptors (5-HT1A, 5-HT1в, 5-HT1d, 5-HT2A, 5-HT2в, 5-HT2c, 5-HT7) causes vasoconstriction of the vessels of the gastrointestinal tract and the central nervous system.
Dopamine receptors are present in both the central nervous system and peripheral organs. Dopamine leads to an increase in peripheral vascular resistance. It increases systolic blood pressure as a result of stimulation of α-adrenoreceptors and increases the strength of heart contractions as a result of stimulation of β-adrenoreceptors.
Acetylcholine is a vasodilator, it dilates blood vessels through muscarinic receptors of the central nervous system, autonomic ganglia, smooth muscles, exocrine glands, heart and alveoli.
Humoral regulation
The paracrine mechanism of hormone action is realized in the brain due to the absence of efferent links. The main mediators are: dopamine, serotonin, GABA, glycine, nitric oxide (NO), O2, CO2. Some hormones, for example, corticosteroids in small amounts are able to penetrate the blood-brain barrier.[11,12]
Humoral regulation includes hormones and biologically active substances (BAS), Picture 2.
The main role in the humoral regulation of the vascular bed is played by hormones of the medulla (catecholamines) and the adrenal cortex (mineralocorticoids, glucocorticoids), the posterior pituitary (vasopressin), pituitary (adrenocorticotropic hormone), thyroid gland (thyroxine, triiodothyronine), angiotensin II, as well as BAS: histamine, serotonin, etc., gases (O2 and CO2), metabolites: lactate, adenosine, ADP.
The renin-angiotensin-aldosterone system (RAAS) plays an important role in the humoral regulation of blood circulation. Its components are renin, aldosterone, angiotensinogen and its derivative forms – angiotensin I and angiotensin II.
Activation of RAAS is initiated by the production of the renin enzyme by juxtaglomerular cells of renal afferent arterioles. Its formation is especially intense in renal ischemia [13-17]. The localization of juxtaglomerular cells makes them particularly sensitive to changes in blood pressure, as well as the concentration of Na+ and K+ ions in primary urine flowing through the renal tubules. The action of factors causing a decrease in fluid volume (dehydration, drop in blood pressure, blood loss, etc.) or a decrease in the concentration of NaCl in the blood and primary urine stimulates the release of renin.
The release of renin is influenced by the state of the central nervous system, as well as a change in body position. When moving from a lying position to a standing position (orthostatic test), renin secretion increases. This reflex reaction is caused by an increase in the tone of the sympathetic part of the autonomic nervous system, transmitting impulses to the beta-adrenergic receptors of juxtaglomerular cells.
The substrate affected by renin is angiotensinogen, a protein belonging to the α2-globulin fraction formed by the liver. The synthesis of angiotensinogen increases significantly under the influence of glucocorticoids and estrogens. As a result of the action of renin, the decapeptide "angiotensin I" is split off from the angiotensinogen – a compound with a weak effect on blood pressure. Under the influence of angiotensin converting enzyme (ACE), the octapeptide "angiotensin II" is cleaved off – a substance with a powerful vasoconstrictor effect. ACE (peptide carboxypeptidase) is an integral protein located mainly on the membrane of endothelial cells, epithelium, mononuclears, nerve endings, cells of reproductive organs, etc. The soluble form of ACE is present in almost all body fluids.
It should be noted that ACE not only leads to the formation of angiotensin II, but also destroys bradykinin, a compound with vasodilatory properties. Therefore, an increase in blood pressure when exposed to ACE is associated not only with the formation of angiotensin II, but also with the breakdown of bradykinin.
Angiotensin II has a powerful vasoconstrictor (vasoconstrictor) effect, significantly superior in strength to norepinephrine, but unlike the latter, it does not cause the release of blood from the depot. This is due to the presence of angiotensin-sensitive receptors only in precapillary arterioles, which are located unevenly in the body, and therefore its effect on the vessels of different areas is not the same. The systemic pressor effect is accompanied by a decrease in blood flow in the kidneys, intestines, skin and an increase in the brain, heart and adrenal glands. Changes in blood flow in the muscle tissue are insignificant. Large doses of angiotensin can cause narrowing of the blood vessels of the heart and brain.
In addition to its direct effect on the vascular system, angiotensin has an indirect effect through the autonomic nervous system and endocrine glands. It increases the secretion of adrenaline, norepinephrine and aldosterone, enhances vasoconstrictor sympathetic effects.
Mineralocorticoids (aldosterone, deoxycorticosterone) are produced in the glomerular layer of the adrenal cortex. They have the ability to enhance the reverse absorption of sodium in the kidneys, salivary glands, digestive tract, increase the sensitivity of vascular walls to the action of catecholamines (adrenaline and norepinephrine).
Glucocorticoids – hormones of the bundle layer of the adrenal cortex increase the sensitivity of vascular adrenoreceptors to catecholamines, enhance the pressor effect of angiotensin-IIThey reduce capillary permeability, enhance myocardial contractility, have a weak mineralocortic-like effect, increase the formation of NH3, which tones the vasomotor center.
Vasopressin (neurohypophysis hormone) causes narrowing of the arteries and arterioles of the abdominal cavity and lungs. However, as under the influence of adrenaline, the vessels of the brain and heart respond to this hormone by expanding, which helps to improve the nutrition of nervous tissue and heart muscle.
Biologically active substances (histamine, serotonin, bradykinin), local hormones (prostaglandins), adenosine, lactate have the ability to dilate blood vessels, many of which are involved in the implementation of local regulatory mechanisms. Histamine causes dilation of most vascular regions by stimulating the release of NO and inhibiting the release of norepinephrine from sympathetic nerve endings and constriction of a number of vessels with direct action.
In nervous and humoral regulation, hemodynamic mechanisms of short-term action, intermediate and long-term action are distinguished.[12]
The mechanisms of short–term action include reactions of nervous origin -baroreceptor, chemoreceptor, reflex to CNS ischemia. Their development takes place within a few seconds.
Intermediate (in time) mechanisms include changes in transcapillary metabolism, relaxation of the vessel wall, and the RAAS reaction. It takes minutes to activate these mechanisms, and hours for maximum development.
Long-acting regulatory mechanisms affect the ratio between intravascular blood volume and vascular capacity. Renal regulation of fluid volume, the action of vasopressin and aldosterone are involved in this process.
In addition, humoral regulation of the tone of the cerebral vessels and cerebral circulation is carried out with changes in the partial voltage of O2 and CO2.
Local mechanisms of regulation of cerebral circulation
Local mechanisms are shown in the diagram.
Local regulation is implemented by metabolic (pH, ADP, adenosine), intravascular, and vascular wall remodeling.[3]
The metabolic regulation is based on the influence of products formed during metabolism (CO2, lactate, ADP, H+) that affect the tone of precapillary arterioles and increase the number of functioning capillaries in accordance with the functional activity of the organ. With increased activity of skeletal muscles, the formation of ATP lags behind its needs, leading to an increase in its decay products – ADP and AMP. However, their excess activates ATP resynthesis in mitochondria and increases oxygen consumption in the cell. The resulting excess of adenosine inhibits the transport of Ca2+ into the smooth muscle cells of arterioles. As a result, their walls relax, tissue blood flow increases, which entails an increase in the oxygen supply to the muscle and an increase in ATP synthesis.
H+ ions, biologically active substances (kinins, prostaglandins, histamine, etc.) also play an important role in the local regulation of blood circulation.
The effect of metabolites produced in tissues on smooth muscle cells is realized according to the principle of negative feedback. An increase in metabolites leads to a decrease in the tone of precapillary sphincters, and their decrease causes a reverse reaction. Low voltage O2 and high – CO2 have similar effects, increasing the concentration of H +.
Intravascular regulation includes:
blood flow–dependent vascular dilation (Schretzenmayr effect);
myogenic regulation (Ostroumov-Bayliss effect);
mechanisms of chronic autoregulation (vascular remodeling);
endothelial modulation of vascular tone.
The Schretzenmire Effect
The essence of this effect is that in response to an increase in shear stress in the vessel (friction of the flow of blood moving through the vessel), its dilation occurs, and in response to a decrease, vasoconstriction. The Schretzenmayr effect may be important in the blood supply to the brain in pathological conditions accompanied by the formation of turbulent flows (aneurysm, etc.).
In addition to autoregulation of blood flow, the protection of the brain from high blood pressure and excessive pulsation occurs mainly due to the structural features of the vascular system of this area. The peculiarities are that there are numerous bends ("siphons") along the course of the vascular bed. Bends smooth out pressure drops and the pulsating nature of blood flow. With increased activity of the whole organism, for example, in conditions of emotional arousal or excessive physical exertion, cerebral circulation increases by 20-25%. However, such shifts do not cause pathology due to the fact that the brain is the only organ whose main vascular pool is located on the surface of the organ, represented by the vascular system of the soft meninges. In this case, the space between the brain and the dura is a kind of reserve for additional blood supply. [18,19]
The Ostroumov-Baylis effect
Cerebral blood flow is determined by myogenic autoregulation, in which blood flow is relatively constant over a wide range (60 mmHg – 130 mmHg).
The vessels of the brain respond with a vasoconstrictor reaction of muscle fibers with an increase in blood pressure and a vasodilator reaction with a decrease in it. The trigger factor of myogenic regulation is a change in transmural pressure. The role of this mechanism increases with hemorrhagic stroke and cerebral edema, when the transmural pressure in the wall of the cerebral vessels is significantly changed.
Chronic autoregulation of vessels remodeling
By remodeling, long-term autoregulation of the blood vessels of the body and the brain, in particular, is realized. This mechanism is activated in order to adapt the body to chronic changes in circulatory load (overload by pressure, shear pressure, turbulence) and is implemented by changing the structure of the vessel. Vascular remodeling can contribute to the maintenance of local tissue homeostasis, but it can also be falsely adaptive, contributing to the progression of hypertension, atherosclerosis, restenosis. There are several types of vascular remodeling. Dilatory remodeling of the diameter of the arteries (large caliber of coronary arteries in marathon runners, uterine arteries during pregnancy) occurs when the synthesis of endothelial NO is increased for a long time, and constrictor remodeling of the diameter is observed in response to a chronic decrease in blood flow (severe cardiovascular insufficiency, etc.), when the synthesis of endothelial NO is below normal.[8]
With atherosclerotic stenoses and restenoses, neointima hyperplasia is possible. In addition, with atherosclerosis, the growth of collaterals from existing capillaries may increase with the formation of media, adventitia.
A number of factors modulating vascular growth are involved in vascular remodeling reactions (fibroblast growth factor – FGF, vascular endothelial factor – EGF, platelet-produced factor – PDGF, etc.).
Under normal conditions, smooth muscle cells exhibit relative refractoriness to growth stimuli. It is assumed that this is provided by the normal function of endotheliocytes.[20]
Nitrogen oxide (NO) is of particular importance in the mechanisms of antiproliferative action of the endothelium. According to some data, even a single injection of a nitric oxide donor suppresses the proliferation of vascular smooth muscle cells in the area of their damage.
The effectors of the regulation of cerebral blood flow are the main intracerebral arteries and the arteries of the soft meninges, which are characterized by specific functional features.
Endothelial modulation of vascular tone
Analysis of the literature data has shown the important role of the endothelium in the implementation of both systemic and local mechanisms of regulation of vascular tone and cerebral blood flow Of the many functions of the endothelium, the modulation of basal muscle tone is the most significant.
The vasoregulatory function of the endothelium is carried out by several mechanisms: the formation of vasodilators, vasoconstrictors, as well as by the metabolism of vasoactive substances (Picture 7).
In the endothelium, vasodilators (NO, prostacyclin, endothelium hyperpolarizing factor, sodium-uretic peptide type C, adrenomedulin, prostacyclin I2 (PgI2)) and vasoconstrictors are synthesized: endothelin, thromboxane A2 (TxA2), von Willebrand factor (vWF), platelet activation factor (PAF), prostaglandin F2a, thrombin, norepinephrine, endoperoxides, leukotrienes, etc.
Endothelin, as one of the vasoconstrictor agents, is synthesized from a precursor (proendothelin) and released without storage.
Along with the synthesis of vasoactive substances, the endothelial cell captures serotonin and norepinephrine from the blood plasma and destroys them. Ectonucleotidases cleave adenosine triphosphate to adenosine. In addition, an increase in the level of cytosolic Ca2+ initiated through type 1 receptors (α1-adrenergic, peptide) activates calmodulin-dependent NO synthase (NOS) and causes the formation of nitric oxide (NO) by converting L-Arginine to L-citrulline.
As described earlier, NO diffuses to smooth muscle cells, activates soluble guanylate cyclase. Guanylate cyclase synthesizes cyclic guanosine monophosphate (cGMP), an active intracellular mediator that regulates the work of membrane ion channels, protein phosphorylation processes (via protein kinases) and phosphodiesterase activity (Picture 10). As a result, the production of cGMP – a secondary messenger increases, which contributes to a decrease in the concentration of cytosolic Ca2+ in myocytes, leading to vasodilation.
In addition to binding to iron complexes in cGMP, NA interacts with cytochrome P450, causing its inhibition, and also interacts with non-heme iron and zinc-containing proteins.
Produced by the endothelium, it can reach sympathetic nerve endings in the adventitia of small arteries and arterioles and inhibit the release of constrictor neurotransmitters.
The functions of endothelial NO include vasodilation, inhibition of platelet adhesion and aggregation, thrombosis, leukocyte adhesion to the endothelium, release of mitogens from platelets, migration and proliferation of vascular smooth muscle cells, formation of endothelin-1 and platelet activation factor, biological action of angiotensin-2, etc.
The receptor-mediated increase in the level of cytosolic Ca2+ in endotheliocytes causes the release of arachidonic acid (AC) from the phospholipids of cell membranes by activating phospholipase A2 (FLA2). Substances of vasodilatory nature are formed from AC under the action of cyclooxygenase (COX) and epoxygenase (EOG): prostacyclin PgI2 and endothelium hyperpolarizing factor (endothelium-derived hyperpolarizing factor - EDHF).
It is believed that the role of prostacyclin in the mechanisms of endothelium-dependent vasodilation is insignificant, complementing the role of NO and endothelial hyperpolarizing factor.
Blood flow-dependent dilation is mediated mainly by the release of endothelial NO.
Synthesis of NO from L-Arginine occurs with the participation of three isoforms of NOS: neuronal (nNOS) - type I, macrophage or induced (iNOS) – type II, endothelial (eNOS) – type III.
The positions of systemic and local (metabolic) regulation have been explained within the framework of the endothelium-dependent mechanism. Acetylcholine, histamine, metabolites of purine bases, arachidonic acid, and many other metabolic products circulating in the blood realize their vasoactive properties through NO. It has been established that vasodilation of cerebral vessels in hypoxia is the result of increased formation of NO by endothelial cells, and with NOS blockade, their response to hypoxia decreases.[21]
The role of the vascular endothelium and the current formed in it in mediating the responses of cerebral vessels to CO2 has also been established. The introduction of NOS formation inhibitors also eliminates the response of cerebral vessels to CO2, and intravenous administration of large doses of the substrate NOS L-arginine levels this inhibitory response.
The cerebral blood flow reacts to changes in local metabolism. An increase in the activity of neurons and the consumption of O2 causes local vasodilation. Blood gases, in particular partial pressure of arterial CO2 (PaCO2), significantly affect cerebral blood flow. Hyperventilation causes narrowing of the cerebral vessels as a result of an increase in CO2 excretion and a decrease in PaCO2, which contributes to dizziness. On the other hand, an increase in PaCO2 is the cause of cerebral vasodilation. The change in PaO2 has a small effect, but with severe hypoxia (low PaO2), pronounced cerebral vasodilation occurs.[2,5]
Moderate changes in oxygen tension in arterial blood do not significantly affect cerebral circulation and oxygen consumption in the brain, while severe hypoxia causes cerebral vasodilation.
The change in blood flow in response to extracellular acidosis is also carried out with the participation of NO, which is confirmed by the results of seeding the introduction of NO-synthase inhibitors that reduce the response of cerebral vessels. Thrombin, histamine and ADP formed during platelet aggregation also cause vasodilation dependent on the endothelium, mediated by the production of NO.
References
- Bon, E.I.(2018). Morphological ideas about the cerebral blood flow in rats / E.I. Bon, N.E. Maksimovich // News of biomedical sciences.- V.17(1).- P.56-60.
View at Publisher | View at Google Scholar - M Ohata, U Sundaram, W R Fredericks, E D London, S I Rapoport.(1981). Regional cerebral blood flow during development and ageing of the rat brain. 7237097. Published 1981 Jun;104(2):319-32. doi: 10.1093/brain/104.2.319.
View at Publisher | View at Google Scholar - M L Rots , G J de Borst , A van der Toorn , F L Moll , C W A Pennekamp , R M Dijkhuizen , R L A W Bleys.(2019).
View at Publisher | View at Google Scholar - M Ursino.(1991). A mathematical model of overall cerebral blood flow regulation in the rat. 1937513. Published 1991 Aug;38(8):795-807.
View at Publisher | View at Google Scholar - Gennadii Piavchenko , Igor Kozlov , Viktor Dremin , Dmitry Stavtsev , Evgeniya Seryogina , Ksenia Kandurova , Valery Shupletsov , Konstantin Lapin , Alexander Alekseyev , Sergey Kuznetsov , Alexander Bykov , Andrey Dunaev , Igor Meglinski. Impairments of cerebral blood flow microcirculation in rats brought on by cardiac cessation and respiratory arrest. 2021; 34534405. Published 2021 Dec;14(12):e202100216.
View at Publisher | View at Google Scholar - I B Sokolova , I V Sergeev , D P Dvoretskii.(2016). Influence of High Blood Pressure on Microcirculation in Cerebral Cortex of Young Rats. 26742741. Published 2016 Jan;160(3):298-9.
View at Publisher | View at Google Scholar - David L Thomas , Mark F Lythgoe, Louise van der Weerd, Roger J Ordidge, David G Gadian. (2006).Regional variation of cerebral blood flow and arterial transit time in the normal and hypoperfused rat brain measured using continuous arterial spin labeling MRI.16034369. Published 2006 Feb;26(2):274-82.
View at Publisher | View at Google Scholar - B K Siesjö, L Berntman, B Nilsson.(1980). Regulation of microcirculation in the brain. 6991885. Published 1980 Mar;19(2):158-70. doi: 10.1016/0026-2862(80)90037-0.
View at Publisher | View at Google Scholar - Hiroki Kato , Yasukazu Kanai , Tadashi Watabe , Hayato Ikeda , Genki Horitsugi , Jun Hatazawa.(2019).
View at Publisher | View at Google Scholar - Martijn E van Raaij , Liis Lindvere, Adrienne Dorr, Jianfei He, Bhupinder Sahota, F Stuart Foster, Bojana Stefanovic. (2012).
View at Publisher | View at Google Scholar - Xiangyang Zhou , Chunnan Lin , Haibo Liang , Jinhua Yang , Zepeng Ni , Yisheng Huang.(2022). Knockdown of sortilin improves the neurological injury and regional cerebral blood flow in rats after subarachnoid hemorrhage. 36179282. Published 2022 Nov 2;33(16):697-704. doi: 10.1097/WNR.0000000000001833.
View at Publisher | View at Google Scholar - Jayyoung Bae , Teo J Shin , Seonghyun Kim , Dong-Hyuk Choi , Dongrae Cho , Jinsil Ham , Marco Manca , Seongwook Jeong , Boreom Lee , Jae G Kim.(2018). The changes of cerebral hemodynamics during ketamine induced anesthesia in a rat model. 29799675. Published 2018 Nov;11(11):e201800081. doi: 10.1002/jbio.201800081.
View at Publisher | View at Google Scholar - Petra Bonova , Miroslav Gottlieb.(2015). Blood as the carrier of ischemic tolerance in rat brain. 25787695. Published 2015 Aug;93(8):1250-7. doi: 10.1002/jnr.23580.
View at Publisher | View at Google Scholar - Thomas Wood , Elisa Smit , Elke Maes , Damjan Osredkar , Mari Falck , Maja Elstad ,(2016). Marianne Thoresen. Monitoring of cerebral blood flow during hypoxia-ischemia and resuscitation in the neonatal rat using laser speckle imaging. 27081159. Published 2016 Apr;4(7):e12749.doi: 10.14814/phy2.12749.
View at Publisher | View at Google Scholar - G I Lobov , I B Sokolova , O P Gorshkova , M E Shvetsova , D P Dvoretskii. (2020).Contribution of Hydrogen Sulfide to Dilation of Rat Cerebral Arteries after Ischemia/Reperfusion Injury.32249400. Published 2020 Mar;168(5):597-601.
View at Publisher | View at Google Scholar - Mimi Wu , Xiaoping Gu , Zhengliang Ma.(2021). Mitochondrial quality control in cerebral ischemia-reperfusion injury. Mol Neurobiol. 58(10):5253-5271.
View at Publisher | View at Google Scholar - Maksimovich, N. E.(2020). Rat brain and its response to ischemia: monograph / N. E. Maksimovich, E. I. Bon, S. M. Zimatkin. Grodno: GrGMU, 240 (in Russian).
View at Publisher | View at Google Scholar - Linda A Allen , Maia Terashvili , Alison Gifford , Julian H Lombard. (2020).Evaluation of Cerebral Blood Flow Autoregulation in the Rat Using Laser Doppler Flowmetry. 32009652. Published 2020 Jan 19;(155). doi: 10.3791/60540.
View at Publisher | View at Google Scholar - Chie Suzuki , Shintaro Kimura , Mutsumi Kosugi , Yasuhiro Magata. (2017).Quantitation of rat cerebral blood flow using 99mTc-HMPAO. 28063322. Published 2017 Apr;47:19-22. doi: 10.1016/j.nucmedbio.2016.12.006.
View at Publisher | View at Google Scholar - Jesse J Liu, Jeffrey S Raskin , Robin McFarlane , Ravi Samatham , Justin S Cetas.(2020). Subarachnoid Hemorrhage Pattern Predicts Acute Cerebral Blood Flow Response in the Rat. 31407068. Published 2020;127:83-89.
View at Publisher | View at Google Scholar - Kevin Lee , Sara Bohnert , Cory Vair , John Mikler , Jeff F Dunn.(2021). Cerebral blood flow and oxygenation in rat brain after soman exposure. 33147512. Published 2021 Jan 1;336:50-56.
View at Publisher | View at Google Scholar

 Clinic
Clinic
