Research Article | DOI: https://doi.org/10.31579/2834-8389/003
Antidyslipidemic Effect of 5-Fluorouracil against Cyclophosphamide induced Dyslipidemia y in Male Albino Rats
1,3 Pharmacology Department, Faculty of Medicine, Sabratha University, Libya
2 Physiology Department, Faculty of Medicine, Sabratha University, Libya
4 Department of Environmental Studies, Institute of Graduate Studies and Research, Alexandria University
5 Department of Cell Biology, Medical Research Institute, Alexandria University
*Corresponding Author: Azab Elsayed Azab. Physiology Department, Faculty of Medicine, Sabratha University, Libya.
Citation: Rabia A M Yahya, Azab Elsayed Azab, Karema El.M.Shkal, Ahmed M. Attia ,Mona A. Yehia. (2022). Antidyslipidemic Effect of 5-Fluorouracil against Cyclophosphamide induced Dyslipidemia y in Male Albino Rats, J. International Journal of Clinical Case Reports, 1(2) DOI: 10.31579/2834-8389/003
Copyright: © 2022 Azab Elsayed Azab. This is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 10 November 2022 | Accepted: 23 November 2022 | Published: 15 December 2022
Keywords: cyclophosphamide;5-fluorouracil;dyslipidemia;combined chemotherapy; antidyslipidemic effect; male albino rats
Abstract
Background: Cyclophosphamide (CPA) is a drug with a wide spectrum of clinical uses. It is commonly used as anticancer and immuno suppressant agent. It induces hyperlipidemia and myocardium damage. 5-FU is widely used in the treatment of a range of cancers. 5-FU in combination with other chemotherapeutic agents improves response rates and survival in breast and head and neck cancers.
Objectives: The present study aimed to evaluate the antidyslipidemic effect of 5-fluorouracil against dyslipidemia induced by cyclophosphamide in male albino rats.
Materials and Methods:
Twenty-eight male adult rats were grouped randomly into four groups (n=5 for each group).
Group II cyclophosphamide (CPA): Cyclophosphamide at a dose of 10 mg/kg day by day through i.p. to rats for 14 days. Group III Fluorouracil (5-FU): 5-Fluorouracil at a dose of 10 mg/kg day by day in saline was given through i.p. to rats for 14 days. Group IV (CPA+5-FU): Rats were given CPA followed by 5-FU at a dose of 10 mg/kg per day (day by day) through i.p. to rats for 14 days. At the end of the experimental period, rats were anesthetized using light ether. Blood samples were taken and prepared for biochemical measurements.
Results:
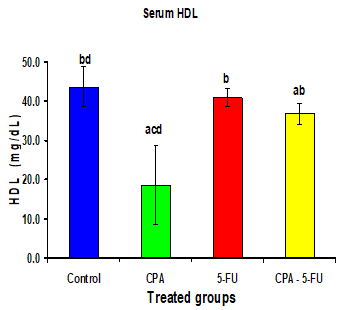
Intraperitoneal administration of CPA or 5-FU resulted in a significant increase in the levels of serum lipids. The cholesterol, Triglyceride, and LDL levels in serum of CPA or 5-FU treated rats were significantly increased when compared to controls. When CPA was administered followed by 5-FU prevented the increase in serum lipid levels highlighting its hypolipidemic role as antagonism. Also, a marked decrease in HDL cholesterol was noted in CPA treated rats. These changes produced by CPA to serum levels of HDL were normalized when 5-FU is given to the treated rats compared to CPA administered rats.
Conclusion:
It could be concluded that treatment of rats with CPA induced dyslipidemia. However, 5-FU and CPA combination could produce a significant amelioration in the dyslipidemic changes, and it may be considered as a potentially useful candidate in the combination chemotherapy with CPA to prevent dyslipidemia.
Future work should consider combined chemotherapy regimens, as two or more mechanisms of action of chemotherapeutic drugs could be more powerful than one mechanism
Introduction
The ultimate clinical effectiveness of any anti-cancer drug requires that it kill malignant tumor cells in vivo at doses that allow enough cells in the patient’s critical tissues (e.g., bone marrow, gastrointestinal tract) to survive so that recovery can occur. This is difficult to accomplish because, in general, anticancer drugs are most useful against malignant tumor with a high proportion of dividing cells, and some normal tissues such as the bone marrow and G1 tract also have a high cell-proliferation rate. Anticancer drugs used by themselves are primarily effective against high-growth-fraction tumors such as the leukemias and lymphomas. The most common malignant tumors, however, are “solid” tumors, including those of the colon, rectum, lung and breast. These tumors usually have a low proportion of dividing cells and therefore are less susceptible to treatment by drugs alone [1]. There are some standard methods of cancer treaments: surgery, chemotherapy, radiation therapy, immunotherapy and biologic therapy. Undoubtedly, chemotherapy and radiotherapy are the treatments to fight cancer with more side effects. Chemotherapy agents can be divided into several categories: alkylating agents (e.g., cyclophosphamide), antibiotics which affect nucleic acids (e.g., doxorubicin), platinum compounds (e.g., cisplatin), mitotic inhibitors (e.g., vincristine), antimetabolites (e.g., 5-fluorouracil), camptothecin derivatives (e.g., topotecan), biological response modifiers (e.g., interferon), and hormone therapies (e.g., tamoxifen). The agents most noted for creating cellular damage by initiating free radical oxidants are the alkylating agents, the tumor antibiotics, and the platinum compounds [2].
Cyclophosphamide (CP) is commonly used as anticancer and immuno suppressant agent. It is mainly used for ovarian cancer, breast cancer, chronic lymphocytic leukemia, non-Hodgkin’s lymphoma, neuroblastoma, soft tissue sarcoma, rhabdomyosarcoma, Wilms’ tumor, and immunosuppressant agent [3, 4]. It induces hyperlipidemia and myocardium damage [4]. Senthilkumar et al. [5] reported that treatment of rats with 150 mg/kg of CPA for 2 days caused a significant alterations in serum cholesterol and none significant changes were observed in serum triglycerides.
CP administration significantly (P<0>) increased the levels of triglyceride, total cholesterol, LDL-cholesterol and decreased the level of HDL-cholesterol in toxic group compared to normal group [4].
5-FU is widely used in the treatment of a range of cancers, including colorectal and breast cancers, and cancers of the aerodigestive tract. Although 5-FU in combination with other chemotherapeutic agents improves response rates and survival in breast and head and neck cancers, it is in colorectal cancer that 5-FU has had the greatest impact. 5-FU-based chemotherapy improves overall and disease-free survival of patients with resected stage III colorectal cancer [6]. The combination of 5-FU with newer chemotherapies such as Irinotecan and Oxaliplantin has improved the response rates for advanced colorectal cancer to 40–50% [7, 8]. However, despite these improvements, new therapeutic strategies are urgently needed.
5-FU has remained the main agent for the treatment of both advanced and early-stage colorectal cancer. Strategies that have been explored to modulate the anticancer activity of 5-FU include decreasing 5-FU degradation, increasing 5-FU activation and increasing the TS binding activity of FdUMP [9].
Despite continuous improvements in cancer therapy and prolonged survival of treated patients, complete remissions and cure of cancer are rare and anti-cancer drugs, which selectively affect tumor cells whilst sparing normal cells, are still being searched extensively.
Objective
The present study aimed to evaluate the antidyslipidemic effect of 5-fluorouracil against dyslipidemia induced by cyclophosphamide in male albino rats.
Materials and Methods
<!-- /* Font Definitions */ @font-face {font-family:"Cambria Math"; panose-1:2 4 5 3 5 4 6 3 2 4; mso-font-charset:0; mso-generic-font-family:roman; mso-font-pitch:variable; mso-font-signature:-536869121 1107305727 33554432 0 415 0;} @font-face {font-family:Calibri; panose-1:2 15 5 2 2 2 4 3 2 4; mso-font-charset:0; mso-generic-font-family:swiss; mso-font-pitch:variable; mso-font-signature:-469750017 -1073732485 9 0 511 0;} @font-face {font-family:"Calibri Light"; panose-1:2 15 3 2 2 2 4 3 2 4; mso-font-charset:0; mso-generic-font-family:swiss; mso-font-pitch:variable; mso-font-signature:-469750017 -1073732485 9 0 511 0;} /* Style Definitions */ p.MsoNormal, li.MsoNormal, div.MsoNormal {mso-style-unhide:no; mso-style-qformat:yes; mso-style-parent:""; margin-top:0cm; margin-right:0cm; margin-bottom:8.0pt; margin-left:0cm; line-height:107%; mso-pagination:widow-orphan; font-size:11.0pt; font-family:"Calibri",sans-serif; mso-ascii-font-family:Calibri; mso-ascii-theme-font:minor-latin; mso-fareast-font-family:Calibri; mso-fareast-theme-font:minor-latin; mso-hansi-font-family:Calibri; mso-hansi-theme-font:minor-latin; mso-bidi-font-family:"Times New Roman"; mso-bidi-theme-font:minor-bidi; mso-ansi-language:EN-US; mso-fareast-language:EN-US;} h3 {mso-style-unhide:no; mso-style-qformat:yes; mso-style-link:"Heading 3 Char"; margin-top:15.4pt; margin-right:0cm; margin-bottom:7.7pt; margin-left:0cm; mso-pagination:widow-orphan; mso-outline-level:3; font-size:13.0pt; font-family:"Calibri Light",sans-serif; mso-fareast-font-family:"Calibri Light"; color:#724128; mso-ansi-language:X-NONE; mso-fareast-language:X-NONE;} p.MsoBodyText, li.MsoBodyText, div.MsoBodyText {mso-style-unhide:no; mso-style-link:"Body Text Char"; margin-top:0cm; margin-right:0cm; margin-bottom:6.0pt; margin-left:0cm; line-height:115%; mso-pagination:widow-orphan; font-size:10.0pt; font-family:"Courier New"; mso-fareast-font-family:"Courier New"; mso-bidi-font-family:"Calibri Light"; mso-ansi-language:X-NONE; mso-fareast-language:X-NONE;} span.Heading3Char {mso-style-name:"Heading 3 Char"; mso-style-unhide:no; mso-style-locked:yes; mso-style-link:"Heading 3"; mso-ansi-font-size:13.0pt; mso-bidi-font-size:13.0pt; font-family:"Calibri Light",sans-serif; mso-ascii-font-family:"Calibri Light"; mso-fareast-font-family:"Calibri Light"; mso-hansi-font-family:"Calibri Light"; mso-bidi-font-family:"Calibri Light"; color:#724128; mso-ansi-language:X-NONE; mso-fareast-language:X-NONE; font-weight:bold;} p.Pa2, li.Pa2, div.Pa2 {mso-style-name:Pa2; mso-style-unhide:no; mso-style-next:Normal; margin:0cm; mso-line-height-alt:10.05pt; mso-pagination:widow-orphan; mso-layout-grid-align:none; text-autospace:none; font-size:12.0pt; font-family:"Calibri Light",sans-serif; mso-fareast-font-family:"Calibri Light"; mso-ansi-language:EN-US; mso-fareast-language:EN-US;} span.BodyTextChar {mso-style-name:"Body Text Char"; mso-style-unhide:no; mso-style-locked:yes; mso-style-link:"Body Text"; mso-ansi-font-size:10.0pt; mso-bidi-font-size:10.0pt; font-family:"Courier New"; mso-ascii-font-family:"Courier New"; mso-fareast-font-family:"Courier New"; mso-hansi-font-family:"Courier New"; mso-bidi-font-family:"Calibri Light"; mso-ansi-language:X-NONE; mso-fareast-language:X-NONE;} .MsoChpDefault {mso-style-type:export-only; mso-default-props:yes; font-family:"Calibri",sans-serif; mso-ascii-font-family:Calibri; mso-ascii-theme-font:minor-latin; mso-fareast-font-family:Calibri; mso-fareast-theme-font:minor-latin; mso-hansi-font-family:Calibri; mso-hansi-theme-font:minor-latin; mso-bidi-font-family:"Times New Roman"; mso-bidi-theme-font:minor-bidi; mso-ansi-language:EN-US; mso-fareast-language:EN-US;} .MsoPapDefault {mso-style-type:export-only; margin-bottom:8.0pt; line-height:107%;} @page WordSection1 {size:612.0pt 792.0pt; margin:72.0pt 72.0pt 72.0pt 72.0pt; mso-header-margin:36.0pt; mso-footer-margin:36.0pt; mso-paper-source:0;} div.WordSection1 {page:WordSection1;} -->
The present research was conducted in the Environmental Toxicology Laboratory, Department of Environmental Studies, Institute of Graduate Studies and Research, Alexandria University, Egypt.
Chemicals
Cyclophosphamide and 5-Fluorouracil were purchased from Sigma Chemical Company (Saint Louis, USA). Chemical Name is 2-[Bis (2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide Cyclophosphamide monohydrate. This substance is listed as a known human carcinogen
Animals
Twenty-eight male adult rats (Sprague Dawley) with an average body weight of 180±10 g were obtained from the Faculty of Agriculture, Alexandria, and acclimatized for two weeks before the experiment. They were assigned to four groups and housed in Universal galvanized wire cages at room temperature (22-25°C) and in a photoperiod of 12h/day. Animals were provided with a balanced commercial diet.
Experiential protocol
Twenty male adult rats were grouped randomly into four groups (n=5 for each group). Group I (control): Rats were injected with saline intraperitoneally at a dose of 1.0 ml/kg b.w. for 14 days. Group II cyclophosphamide (CPA): Cyclophosphamide at a dose of 10 mg/kg day by day through i.p. to rats for 14 days [10]. Group III Fluorouracil (5-FU): 5-Fluorouracil at a dose of 10 mg/kg day by day [11] in saline was given through i.p. to rats for 14 days. Group IV (CPA+5-FU): Rats were given CPA followed by 5-FU at a dose of 10 mg/kg per day (day by day) through i.p. to rats for 14 days.
At the end of the experimental period, rats were anesthetized using light ether. Blood samples were taken from the vena cava of the rat heart. Tubes were used to compile blood drawn from the heart directly; 3 ml of the blood was collected in glass tubes for coagulation and serum formation, blood was allowed to set for 30 min at 4oC to clot, then centrifuged for 5 minutes at 1000 xg. Packed cells were discarded and the supernatant serum samples were decanted and stored into capped sterile polyethylene tubes tubes at -20oC until used (within 24 hours).
Determination of serum cholesterol, triglycerides, LDLc, and HDLc concentrations
Cholesterol was determined after enzymatic hydrolysis and oxidation according to the method described by Richmond [12]. Triglycerides was determined according to the method described by Carr et al. [13].
LDLc Cholesterol test results are based on a reading of light reflected off a test strip that has changed color after blood is applied. The intensity of the color is directly proportional to the concentration of LDLc in the sample. The analyzer converts this reading into a LDLc result and displays it. This test, which selectively measures LDLc, is an enzymatic colorimetric test based on the “Trinder Method” for the determination of cholesterol. In the presence of oxygen, cholesterol is oxidized by cholesterol oxidase to cholesterol-4-en-one and hydrogen peroxide. In the presence of peroxidase, hydrogen peroxide reacts with 4-aminoantipyrine and N, Ndisubstituted aniline to form a blue dye [14].
Lipoproteins are particles comprised of mixture of lipids, phosolipids and apoproteins. There are four distinct groups of lipoproteins such as chylomicrons, very low density lipoproteins (VLDL), low density lipoproteins (LDL) and high density lipoproteins (HDL). The HDL-cholesterol was determined by enzymatic colorimetric method. In this method, Phosphotungstic acid and magnesium ions selectively precipitating all lipoproteins except the HDL-cholesterol fraction cholesterol present in the supernatant can be determined by the same method used for total cholesterol [15].
Statistical Analysis
The values are expressed as mean ± SEM. All values are expressed as mean±standard error of the mean (SEM). The Kolmogorov-Smirnov test was used to assess the normality of distribution of continuous variables. Comparisons between the treatment groups and pathogenic control group were performed by analysis of variance (ANOVA) followed by the Tukey- test. P<0>
Results
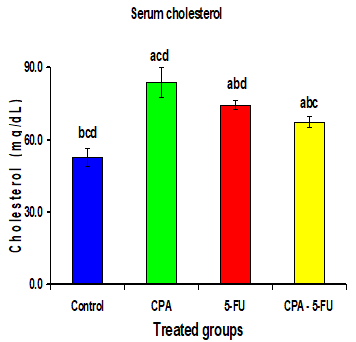
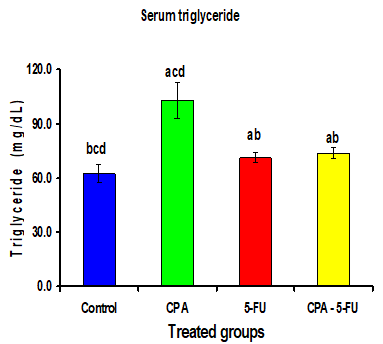
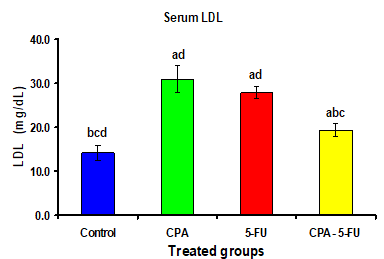
Intraperitoneal administration of CPA or 5-FU resulted in a significant increase in the levels of serum lipids. The cholesterol, Triglycerides, and LDL levels in serum of CPA or 5-FU treated rats were significantly increased (P<0>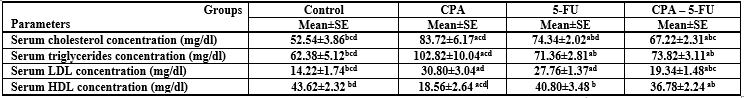
Significance at P > 0.05. CPA: cyclophosphamide; 5-FU: Fluorouracil, a Comparison of control and other groups; b Comparison of CPA
and other groups; c Comparison of 5-FU and other groups; d Comparison of CPA – 5-FU and other groups.
Discussion
Serum cholesterol or its fractions like low density lipoproteins (LDL), high density lipoproteins (HDL) content have been found responsible for many diseases. Cholesterol and lipoprotein levels correlate well with the risk of cardiovascular diseases [17]. Stress in the form of starvation was found to increase lipid peroxidation and alter lipid profile in rabbits [18].
Intraperitoneal administration of CPA or 5-FU resulted in a significant increase in the levels of serum lipids. The cholesterol, Triglyceride and LDL levels in serum of CPA or 5-FU treated rats were significantly increased (P<0>
The metabolism and physiology of lipids and lipoproteins is a dynamic integrated process. Lipoprotein abnormalities resulting in the disruption of serum and cellular lipid levels account for the genesis of vascular diseases. The acrolein-lysine adducts detected in the aorta and plasma LDL of cyclophosphamide treated animals suggest that these adducts wherein acrolein is a metabolite of cyclophosphamide, may play a role in the development of atherosclerosis or atherogenesis [19]. Cyclophosphamide is known to result in hypertriglyceridemia and hypercholesterolemia, which are well known risk factors in cardiovascular diseases [20].
Cyclophosphamide induced elevation in cholesterol levels could be due to increase in biosynthesis and decrease in its utilization. Cyclophosphamide induces free radicals [21], which may cause cellular cholesterol accumulation, (a) by increasing cholesterol biosynthesis and its esterification, (b) by decreasing cholesteryl ester hydrolysis and (c) by reducing cholesterol efflux [22].
The conversion of cholesterol to bile acids is quantitatively the most important mechanism for degradation of cholesterol. However, McClure and Stupans [23] previously reported that after 7 days following a single dose of cyclophosphamide (200 mg/kg body weight) there was a decrease in cytochrome P450 activity in male rats, which may in turn depress cholesterol 7-hydroxylase activity, the key enzyme in the conversion of cholesterol to bile acids.
Decline in the cardiac phospholipid content with a concomitant increase in the serum could be due to the peroxidation of unsaturated membrane lipids by free radicals in biomembranes and tissues causing the leakage of these lipids into circulation [10].
Cholesterol and phosholipids are carried in plasma by lipoproteins, which are synthesized and secreted by the intestine and liver. HDL is secreted from the liver into the bloodstream. Boren et al. [24] have indicated that cardiac apoB enables the heart to secrete excess lipid in lipoproteins. In plasma, Very Low Density Lipoprotein (VLDL) is degraded into IDL and LDL by the action of the enzyme lipoprotein lipase (LPL) and through the exchange reactions with HDL. LDL serves as a major carrier of cholesterol to extrahepatic tissues. High levels of LDL are associated with an increased risk of cardiovascular disease whereas high levels of HDL afford protection by reverse cholesterol transport to liver.
Previously in cyclophosphamide treated rats, lipid composition showed that HDL cholesterol was very low comparatively to a high VLDL cholesterol [20]. In these animals VLDL was larger than normal, corresponding to triglyceride enrichment [25].
Triacylglycerols are degraded by the LPL to fatty acids, which are the chief sources of energy. LPL is predominantly present in the skeletal muscle, cardiac muscle and adipose tissue. Defective secretion of LPL may contribute to the poor expression of lipolytic activity in the vascular bed and to the occurrence of hypertriglyceridemia during cyclophosphamide treatment.
Simultaneously heart LPL activity was also decreased in CPA treated animals [26]. Due to the alterations in LPL activity, increase in triglycerides was associated with a drop in fatty acid levels in heart of group II cyclophosphamide treated rats. The moderate increase in the rate of triacylglycerol synthesis by the liver contributes to the occurrence of hypertriglyceridemia in cyclophosphamide treated rats [27]. Hypercholesterolemia changes in these rats maybe explained by a marked reduction in the activities of fat splitting enzymes, such as plasma cholesterol acyl trasferase (LCAT) and cardiac LPL. The distortion in lipid levels corroborated with abnormalities in the activities of lipid metabolizing enzymes in cyclophosphamide group. Hypercholesterolemia changes in these rats maybe explained by a marked reduction in the activities of fat splitting enzymes, such as plasma LCAT and cardiac LPL [26]. LCAT is secreted by the hepatocytes and released into the plasma. It converts cholesterol into long chain cholesterol ester on HDL and favors reverse cholesterol transport from tissues to liver. The esterification of cholesterol by LCAT leads to the remodeling of the lipoprotein HDL and results in the formation of large HDL particles that are known to offer protection against coronary artery disease [28].
Conclusion
It could be concluded that treatment of rats with CPA induced dyslipidemia. However, 5-FU and CPA combination could produce a significant amelioration in the dyslipidemic changes, and it may be considered as a potentially useful candidate in the combination chemotherapy with CPA to prevent dyslipidemia. Future work should consider combined chemotherapy regimens, as two or more mechanisms of action of chemotherapeutic drugs could be more powerful than one mechanism.
References
- Pratt, W.B. (1994). The Anticancer Drugs, 2ed. New York: Oxford Univ. Press USA, 1994.
View at Publisher | View at Google Scholar - Lamson, D.W. and Brignall, M.S. (1999). Antioxidants in cancer therapy; their actions and interactions with oncologic therapies, Altern Med Rev 4(5): 304-329.
View at Publisher | View at Google Scholar - Chu E, Sartorelli AC. Cancer chemotherapy. In: Katzung BG, and Trevor AJ, editors. (2015). Basic and clinical pharmacology. 13th Ed. New Delhi: McGraw-Hill Education. PP.918-945.
View at Publisher | View at Google Scholar - Das, R., Khuraijam, S. D., Dutta, S., Das, A., Das, P., and Devi, K. K. P. (2017). Effects of Ipomoea aquatica Forsk. In cyclophosphamide induced dyslipidaemia in albino rats. Int. J. Basic Clin. Pharmacol., 6:2743-2748.
View at Publisher | View at Google Scholar - Senthilkumar, S., Devaki, T., Manohar, N.M. and Babu, M.S. (2006). Effect of squalene on cyclophosphamide-induced toxicity. Clinica Chimica Acta, 364(1–2): 335-342
View at Publisher | View at Google Scholar - IMPACT (1995). Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 345: 939–944.
View at Publisher | View at Google Scholar - Douillard, J., Cunningham, D., Roth, A. D., Navarro, M., James, R. D., Karasek, P., and Rougier, P. (2000). Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. The Lancet, 355(9209): 1041-1047.
View at Publisher | View at Google Scholar - Giacchetti, S., Perpoint, B., Zidani, R., Le Bail, N., Faggiuolo, R., Focan, C., and Levi, F. (2000). Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracilleucovorin as first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 18: 136–147.
View at Publisher | View at Google Scholar - Johnston, P. G. and Kaye, S. (2001). Capecitabine: a novel agent for the treatment of solid tumors. Anticancer Drugs 12: 639–646.
View at Publisher | View at Google Scholar - Muralikrishnan, G., Amalan Stanley, V. and Pillai, K. (2001). Dual role of vitamin C on lipid profile and combined application of cyclophosphamide,methotrexate and 5-fluorouracil treatment in fibrosarcoma-bearing rats. Cancer Lett. 169: 115–120.
View at Publisher | View at Google Scholar - Subramaniam S, Shyamala Devi CS (1995) Vitamin E protects intestinal basolateral membrane from CMF-induced damages in rat. Indian J Physiol Pharmacol., 39:263–266
View at Publisher | View at Google Scholar - Richmond, W. (1973). Preparation and properties of a cholesterol oxidase from Nocradia sp. and its application to the enzymatic assay to total cholesterol in serum. Clin. Chem., 18: 1350.
View at Publisher | View at Google Scholar - Carr, T., Andressen, C.J. and Rudel, L.L. (1993). Enzymatic determination of triglyceride, free cholesterol and total cholesterol in tissue lipid extracts. Clin. Chem., 26: 39-42.
View at Publisher | View at Google Scholar - Assmann, G., Jabs, H.U., Kohnert, U., Nolte, W. and Schriewer, H. (1984). LDL- cholesterol determination in blood serum following precipitation of LDL with polyvinylsulfate. Clin. Chem. Acta., 140: 77-83.
View at Publisher | View at Google Scholar - Burstein, M., Scholnick, H.R. and Morfin, R. (1980). Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. Scand. J. Clin. Lab. Invest. 40: 583-595.
View at Publisher | View at Google Scholar - Howell, D.C. (1995): Fundamental statistics for the behavioral sciences (3rd ed.). Duxbury press. An imprint of Wads Worth publishing company Belmont. California. pp. 163-166.
View at Publisher | View at Google Scholar - Eliot RS (1974). Stress and the heart, New York, Futura Publishing Company, 1974; 41-63.
View at Publisher | View at Google Scholar - Lata H, Ahuja GK. and Narang APS, (2002). Effect of starvation stress on lipid peroxidation and lipid profile in rabbits. Indian J Physiol Pharmacol 2002;46: 371-374.
View at Publisher | View at Google Scholar - Arikketh, D., Niranjali, S. and Devaraj, H. (2004). Detection of acrolein-lysine adducts in plasma low-density lipoprotein and in aorta of cyclophosphamide-administered rats. Arch. Toxicol. 78: 397–401.
View at Publisher | View at Google Scholar - Loudet, A.M., Dousset, N., Carton, M. and Douste-Blazy, L. (1984). Effects of an antimitotic agent (cyclophosphamide) on plasma lipoproteins. Biochem. Pharmacol. 33: 2961–2965.
View at Publisher | View at Google Scholar - Lee, C.K., Harman, G.S., Hohl, R.J. and Gingrich, R.D. (1996). Fatal cyclophosphamide cardiomyopathy: its clinical course and treatment. Bone Marrow Transplant. 18: 573–577.
View at Publisher | View at Google Scholar - Gesquiere, L., Loreau, N.,Minnich, A., Davignon, J. and Blache, D. (1999). Oxidative stress leads to cholesterol accumulation in vascular smooth muscle cells. Free Radic. Biol. Med. 27: 134–145.
View at Publisher | View at Google Scholar - McClure, M.T. and Stupans, I. (1992). Investigation of the mechanism by which cyclophosphamide alters cytochrome P450 in male rats. Biochem. Pharmacol. 43, 2655–2658.
View at Publisher | View at Google Scholar - Boren, J., Veniant, M.M. and Young, S.G. (1998). Apo B100-containing lipoproteins are secreted by the heart. J. Clin. Invest. 101, 1197–1202.
View at Publisher | View at Google Scholar - Lespine, A., Dousset, N., Perret, B., De Forni, M., Chap, H. and Douste-Blazy, L. (1988). Accumulation of large VLDL in cyclophosphamide treated rabbits. Relationship with lipoprotein lipase deficiency. Biochem. Biophys. Res. Commun. 154: 633–640.
View at Publisher | View at Google Scholar - Mythili, Y., Sudharsan, P.T., Sudhahar, V. and Varalakshmi, P. (2004). Protective effect of DL-alpha-lipoic acid on cyclophosphamide induced oxidative cardiac injury. European Journal of Pharmacology. 543: 92–96.
View at Publisher | View at Google Scholar - Lespine, A., Azema, C., Gafvels, M., Manent, J., Dousset, N., Chap, H. and Perret, B. (1993). Lipoprotein lipase regulation in the cyclophosphamide-treated rabbit: dependence on nutritional status. J. Lipid Res., 34: 23–36.
View at Publisher | View at Google Scholar - Subramanian, R., Ramaswamy, M. and Wasan, K.M. (2003). Role of lipid and lipoprotein metabolizing enzymes in the development of atherosclerosis. Indian J. Exp. Biol., 41: 14–25.
View at Publisher | View at Google Scholar

 Clinic
Clinic
