Research Article | DOI: https://doi.org/10.31579/2834-5126/073
Adsorption of chromium (vi) and lead (ii) in synthetic solutions using tamarindus indica fruit peel
1Faculty of Chemical Engineering and Agronomy, Universidad de Oriente, Santiago de Cuba, Cuba.
2Industrial Biotechnology Study Center, Faculty of Natural and Exact Sciences, Universidad de Oriente, Santiago de Cuba, Cuba.
*Corresponding Author: Radames Hodelin Barrera., Faculty of Chemical Engineering and Agronomy, Universidad de Oriente, Santiago de Cuba.
Citation: Radames H. Barrera, Taimi B. Horruitiner, Pérez Silva RM, (2024), Adsorption of chromium (vi) and lead (ii) in synthetic solutions using tamarindus indica fruit peel, Clinical Trials and Clinical Research.,3(4); DOI:10.31579/2834-5126/073
Copyright: © 2024, Radames Hodelin Barrera. this is an open access article distributed under the creative commons’ attribution license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 22 July 2024 | Accepted: 16 August 2024 | Published: 23 August 2024
Keywords: bioadsorption; Tamarindus indica; chromium (VI); lead (II)
Abstract
Adsorption allows minimizing the generation of toxic waste and the recovery of the metal. The objective of the work was to study the bioadsorption of Cr (VI) and Pb (II) using the dry peel of Tamarindus indica. We worked at different pH values and concentration levels. The determination of the chemical-physical parameters was carried out at the Empress Geominera Oriente. Adsorption isotherms were performed using the Langmuir, Freundlich and Dubinin-Radushkevich models, resulting in the maximum bioadsorption capacity of Cr (VI) and Pb (II) by biomass being 3.83 and 15.63 mg/g, respectively. reaching maximum removal percentages of 90.8%. The values of mean free energy of adsorption obtained from the Dubinin-Radushkevich model in Cr (VI) and Pb (II) were 10,000 kJ/mol, respectively, showing that, for these experimental conditions, the adsorption process is of a chemical nature.
1.Background
Environmental pollution is a global problem that has attracted the attention of various sectors of the society. Water contamination is utmost important, since this liquid is essential for the life of all living organisms on the planet [1]. In order to reduce the water pollution, some environmental regulations have been established with the objective to regulate the presence of contaminating agents, which have a great incidence in the water quality.
Currently, water pollution with heavy metals is a significant environmental problem. The major sources of heavy metal contaminations are the industrial effluents. Various efforts to reduce the impact of these compounds have been performed. In this sense, some efficient and effective physicochemical and/or biological technologies have been designed for the removal these contaminants. The removal of the metals occurs actively only with living cells and/or passively at the surface of dead cells.
However, low-cost sorbents with high metal binding need to be investigated. Very few information is available on biosorption using agricultural/food bioproducts. [2]
In this study, we used the dry peels of Tamarindus indica to remove the ions Pb (II) and Cr (VI) from the wastewater of the Rente, Fibrocemento, and Galvánica industries of Santiago de Cuba. We also studied the effect of the pH and metal ion concentration in the bioadsorption process.
2.1 Bioadsorption Study
Chemical grade reagents of K2Cr2O7 (MERCK) and Pb (NO3)2 (MERCK) were used to prepare the absorbate synthetic solutions of Cr (VI) and Pb (II), respectively. The pH was adjusted by using either 0.01N NaOH or 0.01N HCl in a pH-meter (PACITONIC-Germany). Solutions of Cr (VI) and Pb (II) at a concentration of 2.5, 10.0, 20.0 and, 40.0 mg/L and 0.2, 2.5, 7.5 and 10.0 (mg/L), respectively, were prepared from a stock solution of 1.0 g/L to study the metal ion concentration. [4]
2.1.1 Bioadsorption process
One gram (per liter) of dry vegetal biomass was mixed by separated with 100 mL of solution of each of the metals at different concentrations and kept under constant agitation for 60 minutes at 150 rpm. Subsequently, the samples were centrifuged at 4500 rpm in a centrifuge (Neofuge 5 Heal Force, China) for 10 minutes. The supernatant solution was filtered with coupled Milipore filters of 0.22 µm (White GSWP, of 20 mm diameter, the filtrates were kept at 4 ºC until use. [6,7]
The concentration of the metals after the bioadsorption process was determined by Inductively Coupled Plasma Atomic Emission Optical Spectroscopy (ICP-AES) in a spectrometer (AMETEK, Germany).
2.1.2 Effect of pH on the bioadsorption of Cr (VI) and Pb (II)
The effect of pH on the bioadsorption of Cr (VI) and Pb (II) by the Tamarindus indica dry biomass was studies and selecting the optimum pH for the concentration study, these pH values were taken according to the optimal percentage removal values [14]. Synthetic solutions of the heavy metals under study were prepared at the aforementioned concentrations at pH values of 2.5, 3.5 and 5.5 units.
2.1.3 Effect of the concentration of the heavy metals on their bioadsorption at different pH.
The bioadsorption of Cr (VI) and Pb (II) by Tamarindus indica dry biomass was carried out in synthetic solutions whose initial concentrations of Cr (VI) were 2.5, 10.0, 20.0 and 40.0 mg/L and 0.2, 2.5, 7.5 and 10.0 mg/L for Pb (II).
2.1.4 Determination of nature of bioadsorption by the dry biomass of Tamarindus indica
2.1.4.1 Langmuir isotherm model
The Langmuir isotherm is a model used to describe the concentration of the metal ion in solution with the amount adsorbed on the surface of the bioadsorbent.
2.1.4.2 The Separation Factor (RL) was determined according to the expression, [10].
2.1.4.3 Freundlich isotherm
To correct the defects of the Langmuir isotherm was used the Freundlich isotherm model, which is applied to describe adsorption processes at multiple sites, regardless of their distribution on heterogeneous surfaces with the interaction between adsorbed species, without presenting an energy barrier.
2.1.4.4 Dubini–Radushkevich isotherm
It is a semi-empirical equation where the adsorption process follows a filling mechanism of the pores of the adsorbent. This model assumes that adsorption has a multilayer character, involving Van der Waals forces, and is applicable to physical adsorption processes [13].
For values of E less than 8 kJ/mol, it is inferred that the bioadsorption process is of a physical nature in which weak electrostatic interaction forces of Van der Waals intervene; E values between 8 and 16 kJ/mol indicate that ion exchange predominates in the bioadsorption process and for E values greater than 16 kJ/mol, then it is considered that bioadsorption is of a chemical nature, with ion exchange predominating. formation of stable bonds between the adsorbent and the metal ions [13].
The Dubinin-Radushkevich equation is used to differentiate the physical adsorption of metal ions from the chemical adsorption [10]. For the application of this model, equations 6 and 7 were used.
2.2 Equations
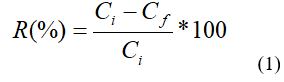
Where: R (%): percentage of removal; Ci (mg/L): initial concentration; Cf (mg/L): final concentration.
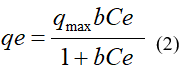
Where: qmax (mg/g): Langmuir constants related to the maximum adsorption capacity of the biomass; b (mg/L): Langmuir constants related to the free energy of adsorption; Ce (mg/L): equilibrium concentration
The constants qmax and b were calculated from the intercept and the slope of the line of best fit to the experimental data, respectively.
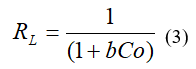
Co (mg/L): initial concentration
b (mg/L): Langmuir constants related to the free energy of adsorption
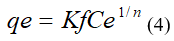
Where: Kf (mg/g) and n are the Freundlich constants and indicate the adsorption capacity and intensity, respectively.
Ce (mg/L): equilibrium concentration
The linear plot of ln (qe) against ln (Ce), will show a straight line if the experimental data corresponds to the model. The constants Kf and n were calculated from the intercept and the slope of the line of best fit to the experimental data, respectively.
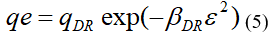
Where: qDR (mmol/g): is the maximum adsorption capacity; βDR (mol2 kJ2): is the activity coefficient related to the free energy of adsorption; ε: is the Polanyi potential
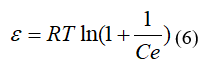
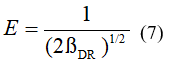
Where: R: is the gas constant, 8.314 kJ/mol; T: is the absolute temperature in degrees Kelvin; E (kJ/mol): is the average free energy of adsorption.
The constants qDR and βDR were determined from the linear plot of ln(qe) vs ε2. The linear plot of ln (qe) against ε2 will show a straight line if the experimental data corresponds to the model. The constants qDR and βDR were calculated from the intercept and the slope of the line of best fit to the experimental data, respectively.[8,9]
3. Materials and Methods
Those peels washed with sufficient distilled water to remove the adhering impurities and dried in the oven at 105 0C for 5 h. They were processed according the established by [3] passed through a sieve to obtain a particle size of 0.84 mm. The sample was placed in a suitable container, labeled as CT-N, and stored until use.
4. Results
When studying this process in detail, the influence of these factors such as pH, adsorbent concentration and metal isotherms in the bioadsorption process must be taken into account [5]
5. Discussion
5.1 Influence of pH on bioadsorption
This is one of the most important variables in the heavy metal removal process, since metal speciation in solution can change depending on this value. In the present study, the effect of pH on the adsorption process of Cr (VI) and Pb (II) by the dry shell of the fruit of the Tamarindus indica plant was determined. The study was carried out at pH 2.5, 3.5 and 5.5 units, in order to know the behavior of the biomass tested in the removal of metals. For Cr (VI), the percentages of removal at pH 2.5 units ranged between 77.9 and 89.8%; at pH 3.5 units between 74.2 and 88.1% and at pH 5.5 units between 60.3 and 74.2?termined by equation 1, (Table 1).
2.4 Tables
pH 2.5 | pH 3.5 | pH 5.5 |
R (%) | R (%) | R (%) |
89.8 | 88.1 | 74.2 |
80.5 | 79.8 | 69.9 |
79.9 | 75.1 | 64.7 |
77.9 | 74.2 | 60.3 |
R (%): percentage of removal
Table 1: Removal of Cr (VI) by the dry shell of the fruit of the tamarind at different Ph.
Regarding Pb (II), the percentages of removal at pH 2.5 units ranged between 83.2 and 90.8%; at pH 3.5 units between 13.0 and 88.1% and at pH 5.5 units between 5.0 and 86.9?termined by equation 1, (table 2).
pH 2.5 5.5 | pH 3.5
| pH
|
R (%) | R (%) | R (%) |
90.8 | 13.0 | 5.0 |
89.5 | 86.8 | 85.7 |
80.9 | 85.5 | 82.5 |
83.2 | 88.1 | 86.9 |
R (%): percentage of removal
Table 2: Removal of Pb (II) by the dry shell of the fruit of the tamarind different pH.
In all cases, at the three pH values studied, a decrease in the percentage of removal can be seen with the increase in pH.
The best removal results are obtained at a pH of 2.5 units for both Cr (VI) and Pb (II). This may be due to the fact that the biomass being worked with is of plant origin and at low pH values the protonation of its surface is activated, which, when positively charged, exerts a strong attraction for the anions HCrO4-, Cr2O72-, CrO42-, Cr4O132- and Cr3O102- [11] which are the most frequent way to find Cr (VI) in solution, which induces an increase in the bioadsorption of this metal. However, when the pH increases, the concentration of OH- ions increases, inducing changes in the surface of the adsorbent, preventing the bioadsorption of negatively charged Cr (VI) ions, which decreases the adsorption of the metal at pH values [12].
With respect to dry biomasses of plant origin, most authors report an optimum pH of 2.0 for Pb (II) adsorption; among these we can mention: eucalyptus bark, bagasse and sugar cane pulp [13] and chemically modified rice husk. With the biomass of Caelsapinia spinosa, they obtained a very low percentage of removal and this is due to the poor selectivity of Pb (II) with respect to functional groups.
5.2 Effect of concentration on bioadsorption of Cr (VI) and Pb (II) at pH 2.5 units
To evaluate the adsorption capacity of Cr (VI) and Pb (II) by the studied biomass, the following conditions were established: adsorbent dose 1.0 g, contact time 60 min, 120 rpm, particle size 0.84 mm and pH 2.5 units chosen by the results obtained in the previous experiment. The concentrations tested they were 2.5, 10.0, 20.0 and 40.0 mg/L for Cr (VI) and 0.2, 2.5, 7.5 and 10.0 mg/L for Pb (II) (see table 3).
Chromium (VI) | Lead (II) | ||||
C0 (mg/L) | Ce (mg/L) | R (%) | C0 (mg/L) | Ce (mg/L) | R (%) |
2.5 | 2.245 | 89.8 | 0.2 | 0.182 | 90.8 |
10.0 | 8.054 | 80.5 | 2.5 | 2.238 | 89.5 |
20.0 | 15.984 | 79.9 | 7.5 | 6.071 | 80.9 |
40.0 | 31.138 | 77.9 | 10.0 | 8.321 | 83.2 |
C0: initial concentration; Ce: equilibrium concentration; R (%): percentage of removal.
Table 3: Percentage of Cr (VI) and Pb (II) removal obtained using the dry shell of the fruit of the tamarind.
The removal percentage for Cr (VI) is between 77.9 and 89.8% and for Pb (II) it is between 83.2 and 90.8%. The highest percentage of removal for Cr (VI) was obtained in the solution with a concentration of 2.5 mg/L and for Pb (II) in the 0.2 mg/L concentration solution. In both cases, a tendency to decrease the removal is observed with the increase in the ion concentration, so that the lower the concentration of the ions, the removal will be more efficient.
According to the composition of the tamarind shell, some of the main components are phenolics, substances of plant origin that have hydroxybenzene functional groups, better known as phenol, linked to aliphatic or aromatic structures, which play an important role in said adsorption. [14].
5.3 Bioadsorption isotherms of Cr (VI) and Pb (II) in aqueous solutions by the dry shell of the fruit of the Tamarindus indica plant
The isotherms allow estimating the amount of adsorbent required, and the sensitivity of the process with respect to the concentration of the product. In the present study, the adsorption isotherms of Cr (VI) and Pb (II) by the dry shell of the fruit of the Tamarindus indica plant were constructed. The Langmuir, Freundlich and Dubinin-
Radushkevich (D-R) adsorption isotherm models were applied to the experimental results obtained in the bioadsorption tests with synthetic solutions, which allow describing the behavior of the bioadsorbent. The parameters obtained for each model are shown in Table 4.
Models | Parametes | Adsorption of Cr (VI) | Adsorption of Pb (II) | ||||
|
| pH 2.5 | pH 3.5 | pH 5.5 | pH 2.5 | pH 3.5 | pH 5.5 |
Langmuir | qm (mol/g) | 3.8252 | 3.2492 | 0.8308 | 15.631 | 7.2524 | 14.328 |
| Kd | 95.26 | 80.15 | 30.18 | 92.43 | 1.09 | 0.38 |
| R2 | 0.9877 | 0.9919 | 0.9994 | 1 | 0.8141 | 0.7999 |
Freundlich | N | 1.348 | 1.3815 | 1.2479 | 1.2013 | 0.3133 | 1 |
| Kf (mol/g) | 4.6531 | 4.5407 | 3.5477 | 4.3229 | 3.0415 | 0 |
| R2 | 0.9909 | 0.9954 | 0.9984 | 0.99 | 0.8089 | 1 |
Dubinin-Radushkevich | qs (mg/g) | 8.3237 | 7.1461 | 6.8452 | 8.2724 | 7.8553 | 7.7967 |
| β(mol2/KJ2) | 5.00E-09 | 6.00E-09 | 6.00E-09 | 5.00E-09 | 5.00E-09 | 5.00E-09 |
| E (kJ/mol) | 10000 | 9128.7 | 9128.7 | 10000 | 10000 | 10000 |
| R2 | 0.9987 | 0.9988 | 0.9988 | 0.9989 | 0.999 | 0.9998 |
Table 4: Parameters of the adsorption models of Cr (VI) and Pb (II) by the dry shell of the fruit of the Tamarindus indica plant.
In the evaluation of the data using the Langmuir adsorption isotherm in its linear form (equation 2), the best values were obtained at pH 2.5 units of qmax (3.83 mg/g) and KL (95.3 mg/g) with a correlation coefficient R2 = 0.99 for Cr (VI) 2.3 Figures
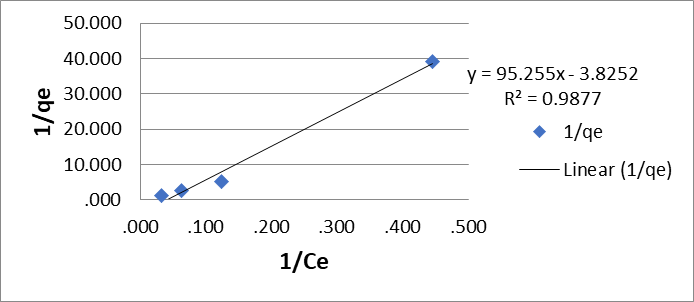
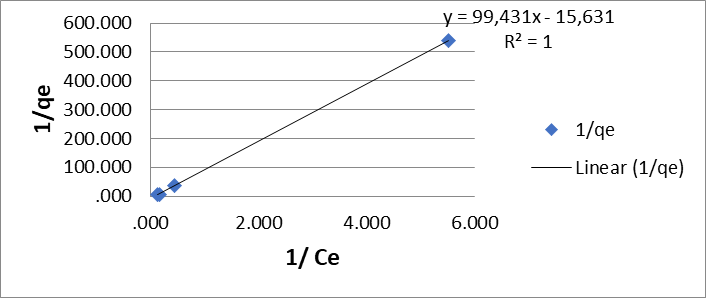
Figure 1: Langmuir biosorption isotherms (A) for Cr (VI) and (B) for Pb (II) by the dry shell of the fruit of the Tamarindus indica plant.
(Figure 1A) and qmax (15.6 mg/g) and KL (92.4 mol/g) with a correlation coefficient R2 = 1 for Pb (II) (Figure 1B).
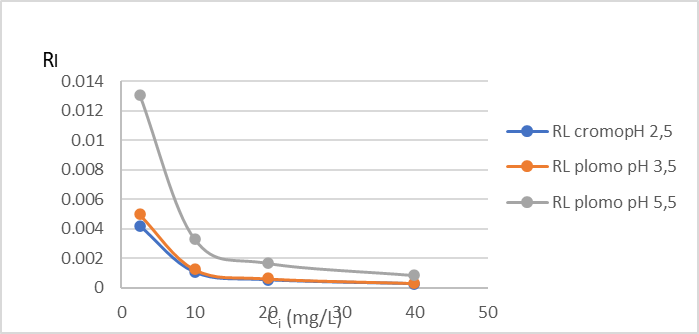
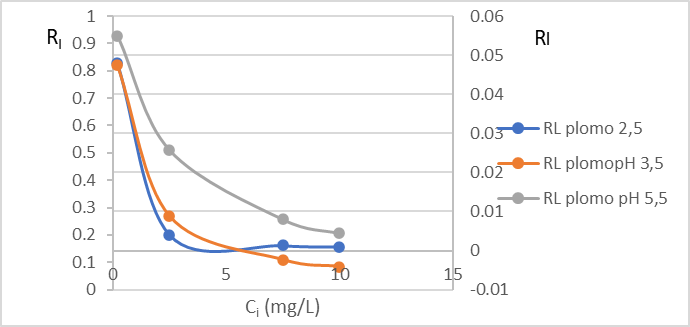
Figure 2: Variation of the separation factor RL (C) of Cr (VI) and (D) Pb (II) by the dry shell of the fruit of the Tamarindus indica plant.
In this study, the separation factor value (RL), determined by equation 3, ranges between 0.00026 – 0.004 for Cr (VI), (Figure 2C) and 0.001 – 0.048 for Pb (II), (Figure 2 D) so we can assume that the adsorption process is favorable.
The Freundlich model is evaluated by equation 4, which is characteristic of a straight line, which allows the determination of 1/n and K on the graph, being 1.35 mol/g and 4.65 mol/g for Cr (VI).
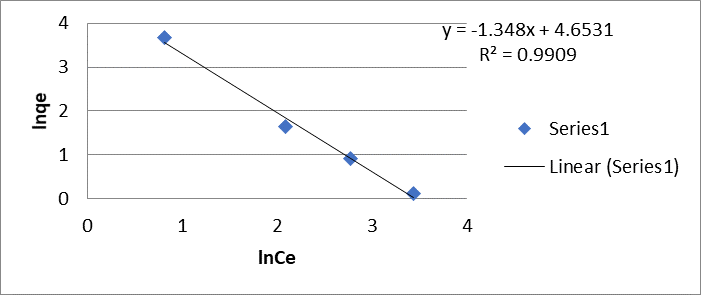
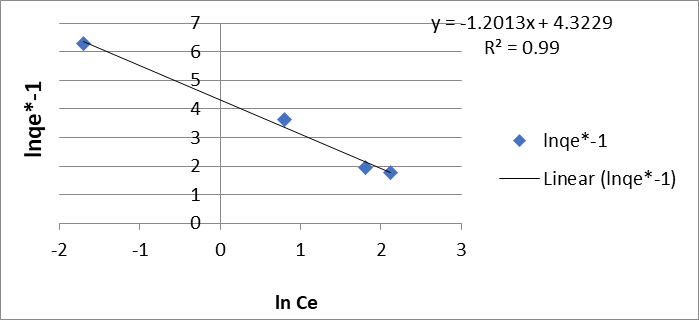
Figure 3: Freundlich biosorption isotherm (E) of Cr (VI) and (F) Pb (II) by the dry shell of the fruit of the Tamarindus indica plant.
(Figure 3 E) and 1.20 mol/g and 4.32 mol/g for Pb (II) (Figure 3 F), respectively.
Taking into account that the reciprocal of n remains at values less than 1, the value obtained is attributed to the heterogeneous nature of the adsorbent surface with an exponential distribution of the energy of the adsorption sites, confirming that the nature of said adsorption it is a chemical process.
The Langmuir and Freundlich isotherms show a linear behavior with correlation coefficients greater than 0.995 for both metals tested, which shows a direct relationship between the concentration of the analyte in the aqueous phase (Ce) and the biomaterial that retains it on its surface.
Comparing our results with other authors, investigated the adsorption of Cr (VI) in aqueous solutions treated with oxalic acid, the maximum removal capacity at pH 3 units of 151.5 mg/g, investigated the use of rice spike as a Cr (VI) bioadsorbent in aqueous solutions at low concentrations, reaching equilibrium in 48 h under normal conditions for a maximum removal capacity of 3.15 mg/g, investigated walnut, hazelnut and almond shells to remove Cr (VI) reaching equilibrium at 100 min, achieving the maximum adsorption values at pH 2.0 and 3.5 units, with values of maximum adsorption capacity 8.01 mg/g for the walnut shell, 8.28 mg/g for hazelnut and 3.40 mg/ g with almond. Investigated the bioadsorption of Pb (II) from wastewater using tomato peel from the Solanum betaceum tree with a maximum capacity of 35.97 mg/g.[15,16]
On the other hand, the Dubinin-Radushkevich adsorption isotherm (D-R) (equations 5 and 6) in its linear form, shows a graph of lnqe vs. ε2 from which the maximum amount of adsorbed metal and the average adsorption energy are obtained, of 8.32 mol/g and 10000 kJ/mol for Cr (VI)
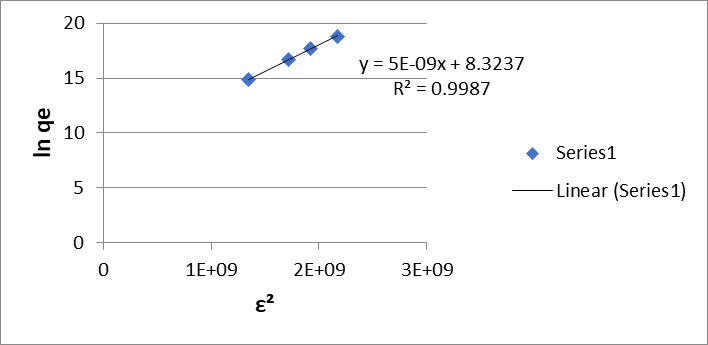
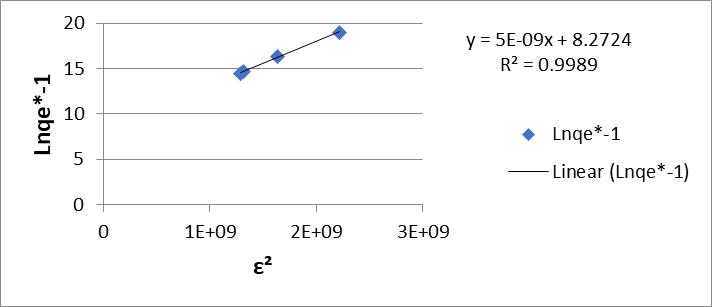
Figure 4: Dubinin-Radushkevich (G) isotherm of Cr (VI) and (H) Pb (II) for the dry shell of the fruit of the Tamarindus indica plant.
(Figure 4 (G)) and 8.27 mol/g and 10000 kJ/mol for Pb (II) (Figure 4 (H)).
The average adsorption energy (E) was evaluated using equation 7. The value obtained was greater than 16 kJ/mol, so it is inferred that the bioadsorption process is chemical in nature.
The adsorption capacity of a biomass depends on several factors, among which are the pH of the solution, the nature of the biomass and the ion to be adsorbed [17,18]
6. Conclusions
The highest maximum adsorption capacity was 3.83 mg/g for Cr (VI) and 15.63 mg/g for Pb (II). The removal percentages were 89.8% at 2.5 mg/L for Cr (VI) and for Pb (II) 90.8% at 0.2 mg/L concentration at pH 2.5 units, respectively. The average adsorption energy values obtained from the Dubinin-Radushkevich model were 10,000 kJ/mol for both metals, which infers that the bioadsorption process is chemical in nature.
Funding
This work is not supported by any external funding
Data Availability Statement
The data supporting the outcome of this research work has been reported in this manuscript.
Conflicts of interest
There is no conflict of interest.
References
- Acosta I, Sandoval P, Bautista D, Hernández N, Juan F, Martínez V M (2012). Bioadsorción de cromo (VI) por la cáscara de mamey (Mammea americana L.). Avances en Ciencias e Ingeniería. 3(2):1 – 9. ISSN: 0718 – 8706.
View at Publisher | View at Google Scholar - Agarwal RM, Singh K (2017). Heavy metal removal from wastewater using various adsorbents a review. Journal of Water Reuse and Desalination. 7(4): 387–419.
View at Publisher | View at Google Scholar - Ahalya N, Kanamadi R D and Ramachandra T V (2008). Biosorption of Chromium (VI) by Tamarindus indica pod shells. Journal of Environmental Science Research International 1(2):77-81. ISO: 690.
View at Publisher | View at Google Scholar - APHA (2021). Standard Methods for the examination of water and wastewater. 23RD Edición. Ed. APHA. Washington D.C. USA.
View at Publisher | View at Google Scholar - Arpita Roy, Navneeta Bharadvaja (2021). Efficient removal of heavy metals from artificial wastewater using biochar. Environmental Nanotechnology, Monitoring & Management. (16).
View at Publisher | View at Google Scholar - Balanta Grande, Danny Carlos David, Zuluaga Fabio (2010). Extracción, identificación y caracterización de quitosano del micelio de Aspergillus niger y sus aplicaciones como material bioadsorbente en el tratamiento de aguas. Revista Iberoamericana de Polímeros. 11(5):297-316. ISSN: 0121-6651.
View at Publisher | View at Google Scholar - Boparai HK, Joseph M, O. Carroll DM (2011). Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. Journal Hazardous Materials. (186):458-465. ISSN: 0304-3894.
View at Publisher | View at Google Scholar - Deepika Thilakan, Jaie Patankar, Srushti Khadtare, Nilesh S Wagh, Jaya Lakkakula, et al. (2022). Plant-Derived iron nanoparticles for Removal of Heavy Metals. International Journal of Chemical Engineering.
View at Publisher | View at Google Scholar - Esmaeili A, Aghababai Beni (2014). A novel fixed-bed reactor design incorporating an electrospun PVA/chitosan nanofiber membrane. Journal of Hazardous Materials. (280):788-796. ISSN: 0304-3894
View at Publisher | View at Google Scholar - Esmaeili A and Aghababai Beni A (2015). Biosorption of nickel and cobalt from plant effluent by Sargassum glaucescens nanoparticles at new membrane reactor. International Journal Environmental Science Technology. (12):2055-2064.ISSN: 1735-1472.
View at Publisher | View at Google Scholar - Gadd G M, & Griffiths A J (1980). Effect of copper on morphology of Aureobasidium pullulans. Transactions of the British Mycological Society: 74(2):387-392.
View at Publisher | View at Google Scholar - Malone L J (1999). Introducción a la química. Segunda Edición, Editorial Limusa, México D. F. Mikac, N. ISBN: 9789681844387.
View at Publisher | View at Google Scholar - Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK. (2010). Adsorption of hazardous dye crystal violet from wastewater by waste material. Journal of Colloid and Interface Science. (343):463-473.ISSN: 1095-7103.
View at Publisher | View at Google Scholar - Padharipande S, & Kalnake R (2013). Tamarind fruit shell adsorbent synthesis, characterization and adsorption studies for removal of Cr (VI) & Ni (II) ions from aqueous solution. International Journal of Engineering Sciences & Emerging Technologies 4 (2):83-89. ISSN: 2231 – 6604.
View at Publisher | View at Google Scholar - Razali N, Mat-Junit S, Abdul-Muthalib A F, Subramaniam S, & Abdul-Aziz A (2012). Effects of various solvents on the extraction of antioxidant phenolics from the leaves, seeds, veins and skis of Tamarindus indica L. Food Chemistry (131): 441–448.
View at Publisher | View at Google Scholar - Sharma D C, & Forster C F. (1994). A preliminary examination into the adsorption of hexavalent chromium using low-cost adsorbents. Biores. Technol. (47):257-264.
View at Publisher | View at Google Scholar - Shen X, J Zhao N, Bonet García E, Villagrasa A, Solé X, et al. (2023) Insights of microorganism’s role in rice and rapeseed wastes as potential sorbents for metal removal. International Journal of Environmental Science and Technology 20 (1): 801 - 814.
View at Publisher | View at Google Scholar - Wang T, Jin Z Chen, M Megharaj and R Naidu. (2014). Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. The Science of the Total Environment (467):10–213.
View at Publisher | View at Google Scholar

 Clinic
Clinic
