Review Article | DOI: https://doi.org/Performance of the reaction to Obtain Biodiesel, F
Performance of the reaction to Obtain Biodiesel, From Used Domestic oils, Using Microwaves as a Heat Source
- Modesto Lorenzo Vega Tang *
- Isidoro Valderrama Ramos
- José Carlos Alcántara Campos
- Adolfo Enrique Guerrero Escobedo
- Jorge Luis Mendoza Bobadilla
- Walter Moreno Eustaquio
- Walter Moreno Eustaquio
Escuela de Ingeniería Ambiental, Facultad de Ingeniería Química, Universidad Nacional de Trujillo, Av. Juan Pablo II s/n – Ciudad Universitaria, Trujillo, Perú.
*Corresponding Author: Escuela de Ingeniería Ambiental, Facultad de Ingeniería Química, Universidad Nacional de Trujillo, Av. Juan Pablo II s/n – Ciudad Universitaria, Trujillo, Perú.
Citation: Modesto L Tang, Isidoro V Ramos, José C Alcántara Campos, Adolfo E Guerrero Escobedo ,Jorge Luis M Bobadilla (2024), Performance of the reaction to Obtain Biodiesel, From Used Domestic oils, Using Microwaves as a Heat Source, International Journal of clinical and Medical Case Reports, 3(5); Doi:10.31579/2834-8664/059
Copyright: © 2024, Modesto Lorenzo Vega Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 20 September 2024 | Accepted: 09 October 2024 | Published: 17 October 2024
Keywords: biodiesel, transesterification, microwave, time of exposition , catalyst, waste oil
Abstract
The main objective of this research work is to evaluate the performance of the reaction to obtain biodiesel from used domestic oils, as a function of time and catalyst, using microwaves as a heat source. A biodiesel was obtained that conforms to the technical considerations of National and International Standards; with 92
% yield, 12.62 cetane index, peroxide index of 0.3667, 102.63 iodine index, 3.65 mm2/s viscosity and a calorific value of 90 390 KJ/Kg. This work can serve as a model for the development of new biodiesel fuels that are interested in contributing to mitigate the environmental impact. It stresses the importance of producing an environmentally friendly fuel, mainly using an input with no commercial value, such as discarded domestic oil. In this context, there is the other contribution of this thesis, the ostensible reduction of the biodiesel production time, which normally takes two hours on average, with microwave activation, is obtained in minutes. The novelty of this work consists in using a statistical method to indicate, in a theoretical way, values where we can start the research. Thus, the Two Level Factorial Design is used, obtaining two theoretical values: the microwave exposure time and the percentage of the catalyst. The experimental values were close to these statistical values obtained and indicated in this Thesis. This was of vital importance in the achievement of the proposed objectives.
Introduction
Global warming and the high demand for fuels, induce the search for new alternative energy sources, especially if they are renewable resources, and even better, if recyclable raw materials are used. The constantly increasing costs of environmental care make it a very attractive source, belonging to the waste- to-energy (WTE) category, which has enormouspotential for fuel production. The main inputsfor this type of processesare recycled domestic oils, municipal solid waste, industrial and agricultural wastes.In order to maintain a policy of environmental protection, toxic wastes emitted by an overpopulated world and, each time in continuous growth, force to look for synthesis methods that are less harmful to the environment. Techniques are also being soughtto mitigate the environmental impact.To generate biodieselfrom plants, the oil contained in their seeds must first be obtained, either by mechanical pressing or by chemical extraction using solvents. High oil prices, the crisis in agriculture, low international oil prices, are some of the factors that have contributed to give additional prominence to biodiesel. Sensitizing elements of society, such as the sanitary crisis of lead, the existence of foreign investors interested in producing this fuel in the country, contribute to thisprocess.The virtues derived from substituting, even partially, the oil, imported in its totality, by another fuel, produced in the country, means foreign currency that we pay to third parties versus foreign currency that we choose to leave in the country, which generates jobs and a chain of multiplying effects in the internal economy. There are few signs that there is political will to work on this issue, that there is a market that demands this product, producers capable of generating the raw material and industrialists interested in processing it, but at least there is interest from certain environmentalist sectors..
Problematic Reality
The search for alternative energy sources to oil is not a recent phenomenon in the world.Based on economicissues, the environmental issue was added to it during the oil crisis of the 1970's. International treaties, particularly those related to Climate Change, have reflected pressures from various sectors to research and implement alternative energy sources to oil. International treaties, particularly those related to Climate Change, have reflected pressures from various sectors to research and implement alternative energies to fossil fuels. In the particular case of biodiesel, its discovery was made a century ago and it has been used for years in Europe and North America. The virtues derived from substituting, even partially, oil, imported in its entirety, with another fuel, produced in the country, means foreign currency that we pay to third parties versus foreign currency that we choose to leave in the country, which generates jobs and a chain of multiplying effects in the domestic economy. There are few signs that there is political will to work on this issue,that there is a marketthat demands this product, producers capable of generating the raw material and industrialists interested in processing it, but at leastthere is interest from certain environmentalist sectors.
1.Equipments, Materials And Reagents
Sansung microwave oven, model T 750 Pot of 250 W. Thermometer from 0 to 400°C. Erlenmeyer flasks 250 ml. Glass beakers, Pyrex brand. Small mortar of 10 cm diameter. Decanting funnels. Vigreoux column of 2.54 cm in diameter and 30 cm long. Glass cooler of concentric tubes of 30 cm in length. Pipettes, micropipettes. Watman No. 90 filter paper. Fisher brand. Equipment to determine the calorific value. Calorimetric pump. Engler viscometer equipment. Equipment to determine the flash point (Pensky-Martens test apparatus).
Reagents: Methanol. KOH in pellets. Glacial acetic acid. Metallic iodine. Chloroform. 0.1 N sodium thiosulfate. 1% starch solution. Distilled water.
Experimental Procedure
1.1.Collection of used oil
1.2.The oil used for this experiment is of vegetable origin, collected from kitchen waste, obtaining a total of 3 liters.
1.3.Inputs and reagents used
The raw material used for the production of biodiesel was used domestic oil. The solvent was reagent grade methanol, the catalyst was sodium hydroxide in an amount of 0.7 to 1.5% weight/weight, as supported by the statistical model.
1.4.Conditioning of the microwave equipment
To carry out the experiments, a hole of 2.60 cm in diameter was drilled in the upper part of the microwave oven, a hole large enough to allow the Vigreoux column to pass into the straight condenser and to obtain the first distillate in the decanting funnel, the by-product to be obtained.
1.5.Characteristis of used oil
Parameter | Unit | Used oil |
Density at 15°C | g/cm3 | 0.80 |
Viscosidad A40°C | mm2/s | 8.0 |
Acidity index | mgKOH/g | 0.67 |
Iodine value | mgyodo/g | 190.50 |
Caloric power | MJ/Kg | 39.54 |
Table 01: Analysis carried out at the Chemistry Laboratory-University of Trujillo
1.6.Oil preparation and biodiesel production
First, the used oil was filtered trhoug one Fisher Brand watman No. 90, which It was placed in a 400 mL beaker, weighed 0.63 g of NaOH pellets and, after finely grinding them, placed them in the 400 mL beaker. Then, poured into the beaker 50 mL of pure methanol. Once the mixture was well stirred with a glass rod and transferred to the beaker, There was activated the microwave for a time from 1 to 8 minutes to obtain biodiesel immediately.There have repeated the process several times, varying the time of exposure to the microwave, from 1 to 8 min. See Table of Yields. For this part, with the data obtained, I observed that at 2.0 minutes I obtained better yields.Then, it was established this time of 2.0 min and varied the amounts of catalyst, from 0.7 to 1.5 wt/wt %. Always keeping constant the quantity of oil ( 50.0 g ) and the solvent ( 50 mL CH3OH ), looking for the best ratio of oil to solvent. Thus, with 50.0 g oil, 1.0 ?talyst, was varied the amounts of methanol from 10 to 100 mL.

Figure. 03. Biodiesel purification.
Finally, the oil was purified: The biodiesel obtained was heated at 60 °C to eliminate residual methanol. It was then transferred to a 500 mL separatory funnel to be washed with distilled water and decanted three times, then it was distilled using a Vigroux column, collecting 500 mL of purified biodiesel.
Results
Molar ratio between solvent and catalystMolar ratio (mole of solvent / mole of oil): If we calculate the ratio of solvent to oil moles (6/1), we have : a) Used oil: 50 g oil/(274g/mol) = 0.182 mol. b) The average density of used oil is 0.80 g/mL.
Methanol (CH3OH): 0.182 x 6 = 1.092 mol CH3OH, 1.092 mol CH3OH x ( 32 g /mol CH3OH ) = 34.94
g Calculating, the volume is approximately 44.00 mL. Catalyst: 0.74 % x ( 84.94 g solution ) = 0.63 g NaOH. To determine the solvent / oil ratio, the percentage of KOH constant
| CH3OH (mL) | CH3OH (g) | CH3OH (mol) | Waste oil (**) (g) | Waste oil (mol) | Relation: Mol CH3OH / Oil mol | Yield (%) |
| 14.75 | 11.68 | 0.3650 | 50.0 | 0.1825 | 2,00 /1.00 | 86.85 |
| 22.12 | 17.52 | 0.5475 | 50.0 | 0.1825 | 3,00 /1.00 | 88.33 |
| 29.49 | 23.36 | 0.7300 | 50.0 | 0.1825 | 4,00 /1.00 | 88,70 |
| 36.87 | 29.20 | 0.9125 | 50.0 | 0.1825 | 5,00 /1.00 | 90.05 |
| 40.55 | 32.12 | 1.0037 | 50.0 | 0.1825 | 5,50 /1.00 | 91.02 |
| 44.12 | 34.94 | 1.0913 | 50.0 | 0.1825 | 5.98 /1.00 | 91.85 |
| 44.24 | 35.04 | 1.0950 | 50.0 | 0.1825 | 6,00 /1.00 | 91.86 |
| 51.62 | 40.88 | 1,.2775 | 50.0 | 0.1825 | 6,50 /1.00 | 91.02 |
Table02: Molar ratio between solventand catalyst
Source: Own elaboration
( * ) Density of methanol = 0.792 g/mL (**) Molecular weight = 32 g /mol
(***) Average molecular weight = 274 g /mol
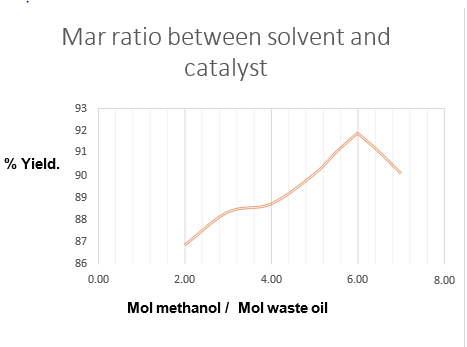
Figure: 04.Molar ratio betweensolvent and catalyst
1.7.Exposure time to microwave vs. performance
| Time (min) | Waste oil (g) | KOH(g) | CH3OH (*) (g) | Yield (%) |
| 1.0 | 50.0 | 0.63 | 34.94 | 86.00 |
| 2.0 | 50.0 | 0.63 | 34.94 | 92.10 |
| 3.0 | 50.0 | 0.63 | 34.94 | 91.50 |
| 4.0 | 50.0 | 0.63 | 34.94 | 91.00 |
| 5.0 | 50.0 | 0.63 | 34.94 | 90.30 |
| 6.0 | 50.0 | 0.63 | 34.94 | 90.00 |
| 7.0 | 50.0 | 0.63 | 34.94 | 88.00 |
| 8.0 | 50.0 | 0.63 | 34.94 | 87.00 |
Table 03: Exposure time to microwave (250 watts) vs. performance
(*) Density of methanol = 0.792 g/mL
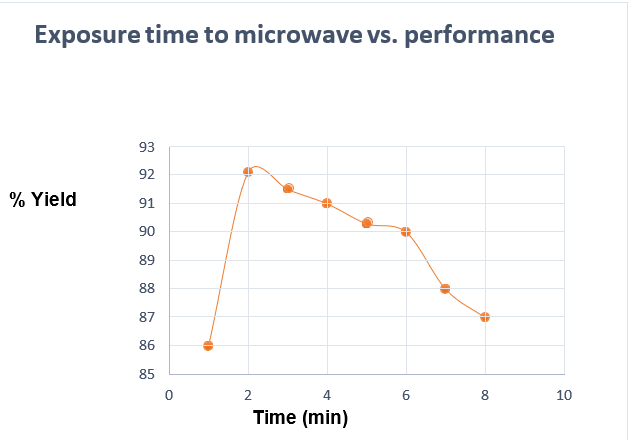
Figure 05. Exposure time to microwave (250 watts) vs. performance1.1.KOH concentration vs. Performance
| KOH (% ) | KOH (g) | Time (min) | Waste oil (g) | CH3OH (g) | Yield (%) |
| 0.7 | 0.5946 | 2.0 | 50.0 | 34.94 | 91.05 |
| 0.8 | 0.6795 | 2.0 | 50.0 | 34.94 | 92.20 |
| 0.9 | 0.7645 | 2.0 | 50.0 | 34.94 | 91.02 |
| 1.0 | 0.8494 | 2.0 | 50.0 | 34.94 | 90.50 |
| 1.1 | 0.9343 | 2.0 | 50.0 | 34.94 | 89.24 |
| 1.2 | 1.0193 | 2.0 | 50.0 | 34.94 | 88.10 |
| 1.3 | 1.1042 | 2.0 | 50.0 | 34.94 | 87.05 |
| 1.4 | 1.1892 | 2.0 | 50.0 | 34.94 | 86.80 |
| 1.5 | 1.2741 | 2.0 | 50.0 | 34.94 | 84.60 |
Table04. KOH concentration vs. Performance
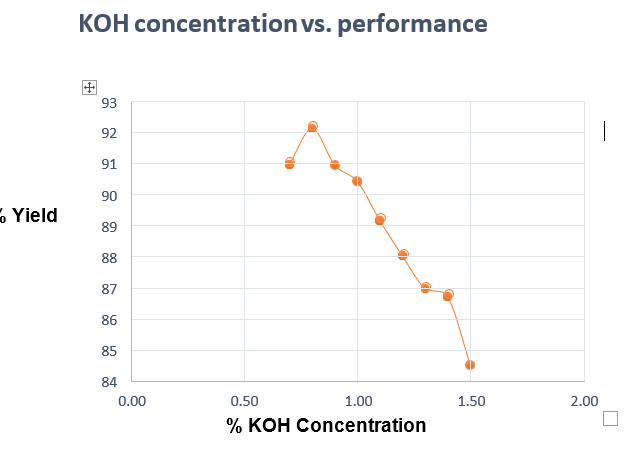
Figure. 06. KOH concentration vs. Performance
Table 05. Comparison of biodiesel parameters obtained with national and international standards
| Parameter | Unit | USA Standar ASTM D6751-07 | Eupean Standar lEN 14214 | Peruvian Standar NTP. 321.003 2005 | Biodiesel obteined for wasteoil |
| VOLATILY | |||||
| Flash point | °C | 130 | 120 | 120 | 120 |
| Temp of destilation (90% recup) | °C | 360 max. | 282 – 360 | 310 | |
| Temp of destilation (95% recup) | °C | 360 max | 320 | ||
| Recovered distillated at 250°C | % vol | 65 max | 15 | ||
| Density at 15°C | g /cm3 | 0.86 – 0.90 | 0.86 – 0.90 | 0.86 – 0.90 | 0.89 |
| FLUENCY | |||||
| Kinematic viscosity at 40°C | mm2/s | 1.9 – 6.0 | 3.5 – 5.0 | 2.0 – 4.5 | 3.65 |
| COMPOSITION | |||||
| Cetane number | 47 min | 51 min | 45 min | 42 min | |
| Acidity index | mg KOH/g | 0.50 max | 0.50 max | 0.08 max | 0.28 |
| Iodine value | Mg Iodine/g | 120 | 102.69 | ||
- Tadistical Data Processing
| Concentration of catalyst ( % | Kind of catalyst | Effect in change of experiment time : 1 to 10 min | Calculated Value (Eti ) |
| 0.7 | A | R2 – R1 = Et1 | -5.90 |
| 1.5 | A | R4 – R3 = Et2 | 1.10 |
| 0.7 | B | R6 – R5 = Et3 | 6.80 |
| 1.5 | B | R8 – R7 = Et4 | -0.70 |
| Summation | 1.30 | ||
| The average time effect in the experiments performed is 1.30 minutes. | |||
Table06. Effect of microwaveexposure time for biodiesel production.
| Concentration of catalyst ( % | Kind of catalyst | Effect in change of 0.7 % to 1.5 % in weight | Calculated Value (Eti) |
| 0.7 | A | R3 – R1 = Ec1 | -1.90 |
| 1.5 | A | R4 – R3 = Ec2 | 1.10 |
| 0.7 | B | R7 – R5 = Ec3 | 2.50 |
| 1.5 | B | R8 – R7 = Ec4 | -0.70 |
| Summation | 1.0 | ||
The averageeffect of the catalyst concentration (KOH) in the experiment is 1.0 % | |||
Table 07. Effect of catalyst for biodiesel production using microwave
3.Discussion
According to the experimental data, the statistical model used suggests a time of 1.30 min. The average effect of the time in the experiments carried out is 1.30 min.
Similarly, the concentration suggested by the statistical model, the concentration of the catalyst used (KOH) in the experiment performed is 1.0 %.
4.Conclusions
1.In this work, it is demonstrated that the most appropriate time for the exposure to the microwave of the reagents and input (discarded oil), at a power of 250 W, is 2.0 min.
2.It is also observed that the most appropriate KOH concentration for this purpose is 0.8 %.
3.A biodiesel can be obtained from recycled domestic oil by the transesterification process using a microwave oven as a heat source; with an acceptable quality, according to National and International Standards.
4.The use of statistics has been of great support to me, since thanks to the two-level factorial design, I was able to save time in the search for the optimum values.
5.The values are close to those indicated by mathematics and statistics.
5.Recommendations
Good control of the reaction temperature is recommended, because an increase of the reaction temperature above 70°C leads to saponification.
Decant by gravity for at least two days, then distill, making sure to remove many impurities that are generated during transesterification.
References
- Akers, M.S. et al. (2006). “Determination of the Heat of Combustion of Biodiesel Using Bomb Calorimetry”, Journal of Chemical Education, Vol. 83, Nª 02.
View at Publisher | View at Google Scholar - Armas Ramírez; Diaz Camacho. (1996) Ciencia Química. Técnicas Experimentales. Edit.Libertad E.I.R.L. Primera Edición, Trujillo-Perú.
View at Publisher | View at Google Scholar - Bankovic – Illic,Ivana;Stamenkovic Olivera, S. (2012) Renewable and Sustainable Energy Reviews . Biodiesel production from non-edible plant oils Vol 16 pág 3621– 3647.
View at Publisher | View at Google Scholar - Box George, Hunter William, Hunter Stuart. (2008). “Estadística para Investigadores. Diseño, innovación y descubrimiento”. Segunda edición. Cap 5 p. 173 – 222. Editorial Reverte S.A.
View at Publisher | View at Google Scholar - Basheer Hasan Diya’uddeen∗, A.R. Abdul Aziz, W.M.A.W. Daud, M.H. Chakrabarti.. (2019) “Performance evaluation of biodiesel from used domestic waste oils”: A review Process Safety and Environmental Protection.Vol 9 0, p. 164–179. (2012).
View at Publisher | View at Google Scholar - CENITC .(2012) .Revista Wear Chech Ibérica. Publicación Norma Europea para combustibles.
View at Publisher | View at Google Scholar - Castellar Rodriguez, Mª Rosario; Obón de Castro Jose Mª. (2009). “Biodiesel a partir de aceites usados”. Universidad Politécnica de Cartagena, Departamento de Ingeniería Química y Ambiental.
View at Publisher | View at Google Scholar - Encinar JM, González JF, Rodríguez Reinares A. (2012). “Biodiesel from used frying oil. Variables affecting the yields and characteristics of the biodiesel”. Ind Eng Chem Res 2005;44(15):5491–5499.
View at Publisher | View at Google Scholar - Immacolata Manco , Laura Giordanib, Vittorio Vaccaria, Massimo Oddonec. (2012) .“Microwave technology for the biodiesel production: Analytical assessments” Fuel. Vol 95.
View at Publisher | View at Google Scholar - Kang-Shin Chen, Yuan-Chung Lin*, Kuo-Hsiang Hsu, Hsin-Kai Wang. (2015) “Improving biodiesel yields from waste cooking oil by using sodium methoxide and a microwave heating system”. Energy Journal. Vol 1, p 104.
View at Publisher | View at Google Scholar - Leadbeater NE, Stencel LM. (2010) “Fast, easy preparation of biodiesel using microwave heating.” Energy Fuels 2006;20(5):2281–2283.
View at Publisher | View at Google Scholar - Leung DYC, Wu X, Leung MKH. (2011) “A review on biodiesel production using catalyzed transesterification” . Appl Energy 2010;87:1083–1095.
View at Publisher | View at Google Scholar - Refaat AA, El Sheltawy ST, Sadek KU. (2018) “Optimum reaction time, performance and exhaust emissions of biodiesel produced by microwave irradiation”. Int J Environ Sci Technol 2008;5(3):315– 322.
View at Publisher | View at Google Scholar - Refaat AA, El Sheltawy ST, Sadek KU. (2010) “Optimum reaction time, performance and exhaust emissions of biodiesel produced by microwave irradiation” . Int J Environ Sci Techno l2008;5(3):315–322.
View at Publisher | View at Google Scholar - Scott J. L, Ratoson C. L. (2000). “Biosesel in new synthesis ”.Journal of Green Chemistry Vol 2, p.245
View at Publisher | View at Google Scholar - Shakinaz A. El Sherbiny, Ahmed A. Refaat, Shakinaz T. El Sheltawy. (2010) Production of biodiesel using the microwave technique”.Journal of Advanced Research. Vol 1, p.309– 314.
View at Publisher | View at Google Scholar

 Clinic
Clinic
