Research Article | DOI: https://doi.org/10.31579/2835-8465/025
Iron Disorder as A Predictor of Complications After Coronary Shunting
- Maksimovich Yelizaveta *
Department of Internal Medicine, Grodno State Medical University, Grodno, Belarus.
*Corresponding Author: Maksimovich Yelizaveta, Department of Internal Medicine, Grodno State Medical University, Grodno, Belarus.
Citation: Maksimovich Yelizaveta, (2026), Considerations on Recurrent Patella Dislocation in the Adolescent Athlete. Orthopaedics Case Reports. 5(1); DOI: 10.31579/2835-8465/025
Copyright: © 2026, Maksimovich Yelizaveta. This is an open-access artic le distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 22 December 2025 | Accepted: 31 December 2025 | Published: 06 January 2026
Keywords: complications; hemolysis; iron disorder; coronary shunting
Abstract
Aim/Purpose: It was to study the role of iron in the development of complications of coronary artery bypass surgery (CABG) in patients with coronary artery disease.
Methods: In accordance with [Hb] In the blood plasma, patients with CABG surgery are divided into 3 groups: 1 – without IOH (Hb≤0.1 g/l), n=43, 2 – with low IOH (lIOH) - Hb>0.1 g/l and <0.5 g/l, n=42, 3 – with high IOH (hIOH) Hb≥0.5g/l, n=38. Determined serum iron - [Fe2+], transferrine (Tr), Tr saturation coefficient (TSC), total serum iron-binding capacity (TSIBC), latent iron-binding capacity of blood, LIBC, ferritin (F) (Mindray BS-200). The complications of cardiovascular (C) genesis were detected. Statistical processing was carried out using the program STATISTICA 10.0.
Results: The most significant differences of the studied parameters at the end of the CABG compared with their initial value, were observed in patients (P) with IOH - an increase in [Fe2+] by 91.9%, TSC – by 87.3%, F – by 165.9% and a decrease in the level of Tr – by 22.0% and LIBC – by 45.0% p<0.001. In the P group with lIOH, the changes were less pronounced (p<0.001), and in the group without IOH there were no changes (p>0.05). The presence of C was observed in 23.6 of P, p<0.001. The highest frequency of C was observed in the group with hIOH (57.9% P, p<0.001), in the group with lIOH – in 5 (11.9% P, p<0.001), and in the group without IOH – only 4.7% P, p<0.001). There were associations (rs) with the frequency of C and TSC – 0.57, F - 0.57, LIBC – -0.56, [Fe2+] – 0.55, Tr – -0.49.
Conclusions: The presence of complications of CABG mainly in patients of the group with the hIOH, the nature of changes in indicators of transport and deposited iron pools, as well as the presence of associations of the frequency of complications with these indicators indicate the involvement of free iron in their development.
Introduction
Coronary artery bypass grafting (CABG) is a highly effective method of myocardial revascularization for coronary artery disease (CAD), significantly improving the prognosis and quality of life for patients. However, despite advances in surgical and anesthetic techniques, CABG is associated with the risk of developing a number of serious postoperative complications, including a systemic inflammatory response, organ reperfusion injury, cardiac arrhythmias, renal dysfunction, and neurological disorders. One of the key factors contributing to their development is the use of a cardiopulmonary bypass (CPB).
Cardiopulmonary bypass, necessary to create a "dry" surgical site, inevitably causes mechanical damage to red blood cells, leading to the development of intraoperative hemolysis (IOH). The release of free hemoglobin into the blood plasma is a direct consequence of IOH and triggers a cascade of pathophysiological reactions. Iron metabolism in the human body is strictly regulated, as iron, an essential component of many enzymatic systems and oxygen transport processes, has a pronounced prooxidant potential in its unbound state. Iron is primarily transported in blood plasma by the protein transferrin, a beta-1 globulin synthesized in the liver. Transferrin effectively binds Fe ions, preventing their toxic effects. Iron is deposited primarily in the form of ferritin, a protein capable of storing thousands of iron atoms in a safe form. Small amounts of ferritin circulate in the serum, reflecting total iron stores and participating in its transport between the reticuloendothelial system and parenchymal cells.
Impaired iron homeostasis and elevated free (highly reactive) iron levels in the serum are known to be associated with the development and progression of cardiovascular diseases, increasing oxidative stress and endothelial dysfunction. Under CPB and IOH, massive hemoglobin release occurs, leading to rapid depletion of buffer systems such as haptoglobin and hemopexin, designed to neutralize it. This results in excess free hemoglobin, which can then be metabolized to release free iron ions. This creates a unique and potentially dangerous situation where the free radical iron pool increases sharply. Despite existing data on the effects of CPB on hemolysis, comprehensive studies examining the dynamics of iron metabolism parameters, particularly in the context of highly toxic free radical iron, depending on the severity of intraoperative hemolysis (IOH) in patients with coronary artery disease after CABG, remain limited or absent altogether. Studying these changes is critical, as the potential increase in free iron levels can exacerbate damage to the myocardium and other organs during ischemia-reperfusion, contributing to the development of postoperative complications and worsening treatment outcomes. In light of the above, this study aims to investigate changes in iron transport and storage pools, as well as free-radical iron markers, in patients with coronary artery disease after cardiopulmonary bypass surgery, depending on the degree of intraoperative hemolysis. The data obtained will allow for a deeper understanding of the pathogenetic mechanisms of postoperative complications and the development of new strategies for their prevention and treatment.
The aim of the study was to investigate the effect of hemolysis on iron transport and iron storage pools in patients undergoing coronary artery bypass grafting.
Materials and Methods
Sources published by readers after surgery: 123 people with unusual speed (groups 1-3). The study corresponded to the principles of the Helsinki Declaration of the World Medical Association "Ethical principles of conducting scientific medical research with human participation" and was approved by the ethical committees of the Educational Institution "GrSMU" and healthcare institutions. City District Clinical Center [http://www.med - pravo.ru/Archives/Helsinki.txt], the information consent is fully from all participants. The study was consistent with the Helsinki Declaration of the World Medical Association «Ethical Principles for Conducting Scientific Medical Research with Human Participation» and was approved by the ethics committees of the Grodno State Medical University and the Grodno Regional Clinical Cardiology Center healthcare institution.
The degree of IOH was assessed by the level of free hemoglobin [Hb] in the blood plasma at the beginning of the operation, immediately after connecting the patient to the artificial device and 15 minutes before removal from the artificial device (Fig. 1), using a HemoCue Plasma / Low Hb analyzer, Sweden [18]. According to [free Hb] in blood plasma, patients who underwent CABG surgery were divided into three groups: Group 1 - without IOH (free Hb ≤ 0.1 g/L), n=43; Group 2 - with low IOH (nIOH) - with [free Hb] >0.1 g/L and <0 n=42; n=38.>0.05) with a predominance of males in all groups (81.3%, p>0.05) [10-12].
Inclusion criteria for the study: patients of both sexes with IHD: Heart failure of II-III degree, indications for IHD, age up to 75 years, informed consent.
Criteria for exclusion from the study: presence of hematological diseases (anemia, leukemia, coagulopathies, thrombophilias), administration of oxygen therapy, erythropoietin or iron preparations, presence of concomitant acute inflammatory and oncological diseases, exacerbation of chronic concomitant pathology, chronic obstructive pulmonary disease, type I and II diabetes mellitus, thyroid pathology, gout, acute and chronic kidney disease, history of acute circulatory disorders (CNS), cognitive disorders that prevent contact with the patient, and refusal to participate in the study.
Results
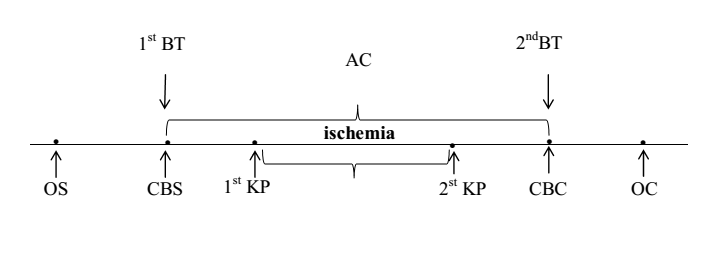
CB – cardiopulmonary bypass
CBS – cardiopulmonary bypass start
CBC – cardiopulmonary bypass completion
KP – cardioplegia
OS – operation start
OC – operation completion
1st BT – first blood test
2nd BT – second blood test
Figure 1: Diagram of the coronary bypass surgery operation
Patients of all groups are comparable by age and gender (Table 1).
Table 1: Clinical characteristics of the subjects
Indicator | Group 1 n=43 | Group 2 n=42 | Group 3 n=38 |
Age, years | 60 (56; 63) | 64 (58; 66) | 66 (60; 68) |
Gender (male), % | 36 (87,8%) | 32 (78,0%) | 31 (78,0%) |
BMI (kg / m2) | 27,8(24,7; 29,2) | 27,7 (24,8; 29,2) | 29,1(25,9; 32,2) |
Total protein (g / l) | 69 (62; 71) | 69(58; 68) | 66(57; 67) |
Glucose, mmol /L | 5,0(4,5; 5,6) | 5,2(4,4; 6,1) | 5,3(4,5; 6,2) |
Cholesterol, mmol /L | 4,1(3,3; 5,0) | 4,6(3,2; 5,7) | 5,0(4,6; 5,6) |
Urea, mmol /L | 5,3(4,8; 5,6) | 6,0(5,5; 7,6) | 6,4(5,5; 7,2) |
Creatinine, mmol /L | 99(89; 104) | 105(98; 110) | 106(99; 112) |
CRP (mg / ml) | 1,2(0,8; 1,4) | 1,1(0,8; 1,3) | 1,0(0,6; 1,2) |
Notes: data are presented as Me [Q25; Q75], where Me is the median, Q25 is the value of the lower quartile;
Q75 is the value of the upper quartile;
All patients underwent surgical intervention using a standard anesthetic protocol under normothermic artificial circulation conditions with a hemodilution level of hematocrit of 25-30%.
The groups did not differ in the duration of artificial circulation and the time of myocardial ischemia (p> 0.05), Table 2.
Table 2: Duration of cardiopulmonary bypass (CB) and myocardial ischemia in patients with varying degrees of IOH during coronary artery bypass grafting
Indicator | Group 1 n=43 | Group 2 n=42 | Group 3 n=38 |
Ischemia-reperfusion time (min) | 69(65; 89) | 74 (68; 78) | 80 (75; 94) |
Ischemia time (min) | 46(39; 64) | 58(56; 62) | 59(51; 68) |
Note: the data are presented in the form Me (Q25; Q75), where Me is the median of the indicator; Q25 - value of the lower quartile;
Q75 is the value of the upper quartile.
Most patients (85%) underwent mammary-coronary bypass surgery in combination with aortic-coronary bypass surgery. Mammary-coronary bypass surgery was performed in 4% of patients (p less than 0.05), aortic-coronary bypass surgery - in 11% of patients (p less than 0.05). Groups were comparable in frequency (p less than 0.05).
More often, lesions of three or more coronary arteries, CA (63.1%) and significantly less often than one CA (7.1%) were revealed, Table 3.
Table 3: Characterization of shunts in patients with coronary heart disease in groups with different levels of IOH
Number of shunts | Group 1 n=43 | Group 2 n=42 | Group 3 n=38 | p1-2 | p1-3 | p2-3 |
1 | 9,9 | 7,5 | 10,8 | 0,412 | 0,510 | 0,314 |
2 | 31,0 | 26,8 | 39,2 | 0,510 | 0,610 | 0,094 |
3 and more | 59,1 | 65,7 | 50,0 | 0,462 | 0,130 | 0,318 |
Left anterior interventricular coronary artery | 87,8 | 100 | 100 | 0,21 | 0,31 | 0,31 |
Left circumflex artery | 4,9 | 7,3 | 19,5 | 0,644 | 0,420 | 0,105 |
Posterior interventricular branch of left circumflex artery | 14,6 | 39,0 | 61,0 | 0,210 | 0,310 | 0,406 |
Left marginal artery | 56,1 | 65,9 | 80,5 | 0,172 | 0,22 | 0,324 |
Right coronary artery | 24,4 | 58,5 | 61,0 | 0,231 | 0,341 | 0,821 |
Right interventricular branch artery | 17,07 | 34,15 | 26,8 | 0,706 | 0,285 | 0,471 |
Accordingly, with myocardial revascularization, three or more coronary arteries (CA) were shunted more often - 56.9% of patients. The most common lesions were observed in the anterior interventricular branch of left coronary artery (p less than 0.05), posterior interventricular branch of the of left circumflex artery (p less than 0.05) and the left marginal artery (p less than 0.05).
Table 4 presents the nosological characteristics of patients.
Table 4: Nosological characteristics of patients with coronary artery disease before coronary bypass surgery with varying degrees of intraoperative hemolysis (IOH)
Indicator | Gr 1 n=43 | Gr 2 n=42 | Gr 3 n=38 |
Ischemic heart disease duration | 8,5 (4,2; 11,4) | 8,9 (4,6; 10,8) | 9,5 (6,2; 12,1) |
Duration of hypertension | 10 (6; 11) | 8 (5; 10) | 11,5 (9; 15) |
Functional class II | 9 (20,1%) | 11 (26,2%) | 6 (15,8%) |
Functional class III | 34 (79,9%) | 31(73,8%) | 32 (84,2%) |
Postinfarction cardiosclerosis | 37 (86,1%) | 36 (85,7%) | 33 (86,8%) |
The number of myocardial infarction (2 MI) in the history | 16(37,2%) | 18(42,8%) | 13(34,2%) |
NYHAII | 36(83,7%) | 31(73,8%) | 33(86,8%) |
NYHAIII | 7(16,3%) | 11(26,2%) | 5(13,2%) |
Ischemic cardiomyopathy | 2 (0,86%) | 3 (1,26%) | 2 (0,76%) |
History of arrhythmias | 7(16,3%) | 7(16,6%) | 6(15,4%) |
Paroxysm of atrial fibrillation | 0 (9%) | 1(0,42) | 1 (0,38%) |
supraventricular extrasystole | 4(1,72%) | 2 (0,84) | 2(0,76%) |
ventricular extrasystole | 1(0,43%) | 1 (0,42%) | 1(0,38%) |
right His bundle branch block | 1(0,43%) | 1 (0,42%) | 1(0,38%) |
left His bundle branch block | 1(0,43%) | 2 (0,42%) | 2(0,76%) |
blood hypertension | 36 (87,8%) | 38(90,2%) | 38(92,7%) |
chronic bronchitis without exacerbation | 7(16,3%) | 9(21,4%) | 12(31,6%) |
gastropathy | 18(41,9%) | 17(40,5%) | 20(52,6%) |
urolithiasis | 6(13,9%) | 9(21,4%) | 7(18,4%) |
osteoarthritis | 0(0%) | 3(7,1%) | 1(2,6%) |
excess BMI and obesity | 36(83,7%) | 31(73,8%) | 33(86,8%) |
excess BMI | 22(51,2%) | 18(42,9%) | 18(47,4%) |
obesity | 14(32,6%) | 13(31%) | 15(39,5%) |
Note: quantitative data are presented in the form Me [LQ; UQ], where Me is the median, LQ is the value of the lower quartile; UQ is the value of the upper quartile, and categorical – in the form of absolute and relative frequencies of signs; for all presented indicators, differences between the studied groups were absent (p> 0.05).
Most patients had one previously suffered myocardial infarction, MI. Patient groups were comparable in the number of MI (p> 0.05), the presence of ischemic cardiomyopathy (p> 0.05) and a history of cardiac arrhythmias (A), p> 0.05. Table 4 presents the frequency and structure of cardiac arrhythmias in patients with varying degrees of intraoperative hemolysis before coronary artery bypass surgery. As you can see, before surgery, cardiac arrhythmias were found in 22 people (17.89%). Among cardiac arrhythmias, AF paroxysms, supraventricular and ventricular extrasystoles, as well as blockade of the right and left legs of the bundle of His were found. At the same time, AF paroxysms were observed in 2 (1.63%) patients, extrasystoles were found in 11 (8.94%), including supraventricular extrasystoles, and in 3 (2,44%) – ventricular extrasystoles were noted. Dysfunction of the conduction function was noted in 8 people (6.5%), including blockade of the left leg of the bundle of His was noted in 5 people (4.07%), blockade of the right leg of the bundle of His - in 3 people (2.44%). The groups were comparable in the frequency and nature of cardiac arrhythmias in the anamnesis (p>0.05).
Patients before CB (1-5 days) and after surgery (within 1-5 days) underwent daily ECG monitoring, as well as standard electrocardiography (ECG). In order to clarify the role of hemolysis in the development of postoperative arrhythmias in the studied groups of patients with different levels of IOH, we analyzed the incidence of cardiac arrhythmias in the perioperative (during the operation and during the first day after it) and in the early period (up to 1 month) and their structure [1].
The examined patients received standard therapy consisting of antiplatelet agents (79.7%), statins (76.4%), beta-blockers (84.6%), angiotensin-converting enzyme inhibitors (76.4%), antianginal drugs (79.7%), table 5.
The drug treatment among patients of the studied groups did not differ in the administration of aspicard and clopidogrel (χ2 = 5.35; p = 0.069), β-blockers (χ2 = 3.18; p = 0.204), but it differed in the reception of statins (χ2 = 12.2; p = 0.006), inhibitors of the angiotensin-converting enzyme, ACE inhibitors (χ2 = 7.13; p = 0.028) and antianginal drugs (χ2 = 13.7; p less than 0.001). In particular, fewer patients in the third group took statins (57.9%, p less than 0.05), inhibitors (63.2%, p less than 0.001) and antianginal drugs (60.5%, p less than 0.001). Patients with a history of cardiac arrhythmias (paroxysmal AF) were treated with antiarrhythmic drugs 5-7 days before surgery.
Table 5: Characterization of drug therapy for examined patients with coronary artery disease before coronary artery bypass surgery with varying degrees of IOH
Index (%) | Gr 1 n=43 | Gr 2 n=42 | Gr 3 n=38 | Gr 1-3 n=123 | χ2 | p |
beta blockers | 86,0 | 90,5 | 76,3 | 84,6 | 3,18 | 0,204 |
ACE inhibitors | 88,4 | 76,2 | 63,2° | 76,4 | 7,13 | 0,028 |
statins | 90,7 | 78,6 | 57,9°• | 76,4 | 12,2 | 0,006 |
antianginal | 93,0 | 83,3 | 60,5°• | 79,7 | 13,7 | 0,0001 |
antiplatelet agents | 88,4 | 81,0 | 68,4 | 79,7 | 5,35 | 0,069 |
Note:
° - p less than 0.05, °° - p less than 0.001– statistical differences with the group without IOH;
• - p less than 0.05, •• - p less than 0.001 - statistical differences with group 2 (with lIOH)
To prevent arrhythmias during the operation, lidocaine was infused in a cardioplegic solution (1-1.5 mg / kg /min). After the operation, antiarrhythmic drugs were administered to arrest AF paroxysm (AF - 5 mg /kg intravenously dropwise for 60 min). Patients after CB took β-blockers (atenolol 25-50 mg / day, metoprolol at a dose of 25-50 mg / day, bisoprolol at a dose of 2.5-5 mg / day) depending on the level of blood pressure. In patients with atrial flutter and ventricular tachycardia in the perioperative period, temporary atrial pacemaker was performed, which was maintained for 72 hours with a frequency of 10 beats / min more than their own heart rate.
Statistical data processing was carried out using the program Statistica 10.0 for Windows (StatSoft, Inc., USA). Given the abnormality of the distribution of attributes, nonparametric methods of descriptive statistics were used for processing: quantitative data are presented in the form Me [LQ; UQ], where Me is the median, LQ is the value of the lower quartile; UQ is the value of the upper quartile; categorical data are presented in the form of absolute and relative frequencies. When comparing the medians of quantitative variables of several independent groups, the Kruskell-Wallis test was used, to compare categorical data, the exact Fisher test, the χ2 criterion, with the Yeats correction at low frequencies were used. The strength of the relationship between the indicators was estimated using a correlation analysis based on the association coefficient (Kendall criterion) by its value (rs ≤0.25 - weak; 0.25 less than rs less than 0.75 - moderate and ≥0.75 - strong). In order to check the dependence of the incidence of arrhythmias on the degree of IOG, determined by the level of free hemoglobin, a logistic regression analysis and ROC analysis were performed in the statistical program SPSS Statistics 21.0 (SPSS, USA). Differences were considered significant at p less than 0.05.
Results studies
The most common complications of CB during myocardial revascularization in patients with coronary artery disease were various types of arrhythmias, which were observed both in the perioperative period and during the month of observation - an early period (Table 6).
Table 6: Frequency and structure of arrhythmias in patients with coronary artery disease after CBS
Types of arrhythmias | Arrhythmia frequency (%) |
p | |||||
all | PP | EP | |||||
n | % | n | % | n | % | ||
Total arrhythmias | 27 | 22,0 | 14 | 11,4 | 13 | 10,6 | NS |
ventricular fibrillation | 3 | 2,43 | 2 | 1,63 | 1 | 0,81 | NS |
ventricular tachycardia | 3 | 3,25 | 3 | 2,43 | - | - | NS |
atrial fibrillation | 7 | 5,70 | 3 | 2,43 | 4 | 3,25 | NS |
atrial flutter | 2 | 1,62 | 1 | 0,81 | 1 | 0,81 | NS |
supraventricular tachycardia | 1 | 0,81 | - | - | 1 | 0,81 | NS |
others: | 11 | 8,9 | 5 | 4,07 | 4 | 3,25 | NS |
AV block 1-2 degree | 2 | 1,6 | - | - | 2 | 1,6 | NS |
ventricular extrasystole | 5 | 4,1 | 4 | 3,25 | 3 | 2,43 | NS |
supraventricular extrasystole | 4 | 3,3 | 1 | 0,81 | 3 | 2,43 | NS |
Note: PP - perioperative period, EP - early period. data are presented in the form of absolute and relative frequencies of signs;
AV block - atrio-ventricular block.
Discussion
In patients with varying degrees of IOH, the transport (serum iron, TIBC, LIBC, transferrin) and deposited (ferritin) iron pool parameters were studied. During surgery, there was an increase in [free Hb] in groups 2 (with nIOH) and 3 (iIOH). In group 1 (without IOH), no changes in [free Hb] were observed (p> 0.05). Moreover, in the group with nIOH, [free Hb] at the end of surgery, compared with the initial level, increased by 6 (2.5; 8.0) times (z = - 5.781, p less than 0.001), in the group with iIOH – by 10 (7; 12) times (z = - 5.59, p less than 0.001). The "serum" iron parameter – [FeSer.] represents the main part of the iron in the blood plasma bound to protein transferrin and characterizes its mobile pool in the body. At the beginning of the CABG surgery, its content in the blood plasma of patients in the iIOG group was 16.45 (14.5; 19.9) μm/L, which did not differ from the [Fesyv.] value in patients in the non-IOG group – 16.42 (14.24; 18.3) μm/L (p=0.44), in the nIOG group – 14.47 (12.8; 16.9) μm/L (p=0.1225), as well as in the comparison group – 14.6 (12.5; 20.6) μm/L (p=0.922) and the “control” group – 16.4 (13.3; 20.8) μm/L (p=0.2889).
At the end of the surgery, [Fesyv.] in the blood of patients in the iIOG group was 29.6 (23.7; 33.0) μm/l, which is higher than in patients without IOG – 15.0 (12.9; 19.3) μm/l (p less than 0.001), as well as in the group with nIOG – 17.9 (15.9; 22.1) μm/l (p less than 0.001), the comparison group – 14.6 (12.5; 20.6) μm/l (p less than 0.001) and the control – 16.4 (13.3; 20.8) μm/l (p less than 0.001). When comparing dependent variables using the Wilcoxon test, it was found that [Fesyv.] in the blood of patients at the end of CABG surgery in the group without IOG did not change (z= -1.223; p=0.223), but decreased compared to the value of the indicator at the beginning of the operation in patients with both low (z= -5.646; p=0.001), and with a high degree of IOH (z=-0.537; p=0.001). The value of transferrin in blood plasma – [Tr] at the beginning of the operation in patients of the group without IOH was 210.0 (196.0; 223.0) μg/ml, not differing from the value of this indicator in the group with nIOH – 204.3 (195.9; 228.0) μg/ml (p=0.5827), the group with iIOH – 202.2 (193.8; 216.0) μg/ml (p=0.1584) and was comparable with the level of [Tr] in patients of the comparison groups – 214.0 (206.0; 225.0) μg/ml (p=0.6831) and the control – 224.3 (205.0; 238.2) μg/ml (p=0.6858). There were also no differences in [Tr] in group 2 (niOG) and in the comparison group (p=0.5508) and in the control group (p=0.5608), as well as in group 3 and in the comparison (p=0.2318) and control (p=0.2211) groups. No differences in [Tr] values were noted between the comparison and control groups (p=0.334).
At the end of CABG, patients with IOG showed a decrease in [Tr]: in the iIOG group - to 150.6 (145.4; 168.0) μg/ml, which is lower than in patients in the nIOG group - 190.0 (174.2; 208.8) μg/ml (p less than 0.001) and the group without IOG - 203.0 (190.0; 220.0) μg/ml (p less than 0.001), as well as comparison (p less than 0.001) and control (p less than 0.001) groups [17]. There were no differences [Tp] between the groups without IOG, comparison (p=0.173) and control (p=0.176).
The Wilcoxon test showed that the blood transferrin level in patients after CABG, compared with the baseline, did not change in the group without IOH (z=-1.615; p=0.106), but decreased in patients with a low (z=-5.646; p less than 0.001) and high degree of IOH (z=-5.373; p less than 0.001). The degree of transferrin saturation with iron reflects the transferrin saturation coefficient (TSC). Its value in the comparison group was 33.0 (27.1; 43.4)%, which did not differ from the value in the control group - 36.0 (27.3; 43.4)% (p less than 0.001), Fig. 3. At baseline, no differences in the CNT were observed in the study groups: the group with iIOG - 40.3 (31.3; 46.0)%, without iIOG - 30.9 (27.3; 42.5)% (p> 0.05), and with nIOG - 34.1 (29.0; 39.9)% (p> 0.05), as well as in the comparison and control groups (p> 0.05).
During the operation, patients with IOH showed an increase in the CNT, as a consequence of an increase in the level of free iron, compared with the value of this indicator at the beginning of CABG, to a greater extent in patients with low IOH - 40.2 (35.8; 55.0)% (z = -5.645; p less than 0.001) and with a high degree of IOH - 65.9 (57.2; 77.3)% (z = -5.330; p less than 0.001). No changes in the CNT were noted in the group without IOH - 34.1 (29.0; 39.9)% (z = -1.639; p = 0.101). Moreover, differences were noted between the CNT indicator in the 3rd group compared to the value in the other studied groups (p less than 0.001), and in the 2nd group - compared to the value in the 4th, 5th and 1st groups, p less than 0.001. Analysis of the dynamics of [TIBC] in blood plasma did not reveal any differences in the indicator between the groups of operated patients either at the beginning or at the end of CABG. At the beginning of the operation, in patients in the group without IOH, [TIBC] was 44.0 (42.8; 45.6) μmol/l (p=0.090), in groups with nIOH – 43.0 (42.1; 43.6) μmol/l (p=0.082), with viIOH – 43.2 (43.9; 44.0) μmol/l, and in the comparison groups – 47.5 (43.4; 47.5) μmol/l (p less than 0.001) and control – 49.1 (45.8; 50.4) μmol/l (p less than 0.001). At the end of the surgery, [TIBC] in the groups of patients undergoing surgery was comparable both among themselves and compared to the baseline level, amounting to 41.2 (38.4; 47.1) μmol/L (p=0.846) in the group without IOH, 44.3 (40.3; 45.6) μmol/L (p=0.621) with nIOH, and 43.9 (40.0; 48.0) μg/ml (p>0.05) with iIOH. The lack of change in [TIBC] in the groups of patients with IOH may be due to a decrease in latent iron-binding capacity due to excessive saturation of transferrin with iron against the background of an increase in serum iron due to hemolysis.
When comparing dependent variables using the Wilcoxon test, the [TIBC] of patients at the end of CABG did not change in the group without IOH (z= -1.595; p=0.111), with nIOH (z= -1.017; p=0.309) and with iIOH (z=-1.102; p=0.271), compared with the baseline value.
The baseline values of [TIBC] in the groups of operated patients were: in the group with iIOH - 27.5 (23.1; 29.6) μmol/L, in the group without IOH - 30.4 (25.7; 32.4) μmol/L (p=0.002), in the group with nIOH - 28.4 (24.8; 30.5) μmol/L, p>0.05 (Figure 4.5). In patients in the comparison group, [LVSS] was 30.8 (27.2; 34.3) μmol/L (p less than 0.001), while in the control group it was 30.4 (27.1; 37.0) μmol/L (p less than 0.001).
A decrease in transferrin levels and an increase in KNT during CABG led to a decrease in [LVSS] in patients in the iIOG group to 14.4 (9.1; 19.3) μmol/L, which is lower than in the non-IOG group – 27.2 (23.4; 30.1) μmol/L (p less than 0.05) and did not differ from [LVSS] in the nIOG group – 26.2 (19.8; 28.1) μmol/L (p>0.05).
In patients with iIOH, there were more significant differences in the studied parameters compared with their baseline values: an increase in [Fe2+] in blood plasma by 91.9 (4.8; 117.5)%, p less than 0.001, KNT – by 87.3 (34.0; 30.4)%, p less than 0.001, ferritin – by 165.9 (135.1; 212.6)% (p less than 0.001) and a decrease in the level of transferrin – by 22.0 (19.7; 30.2)%, p less than 0.001 and LVSS – by 45.0 (31.3; 68.7)% p less than 0.001.
Conclusion
Our study revealed significant patterns in the dynamics of the transport and storage pools of iron in patients undergoing CABG using CPB, depending on the degree of intraoperative hemolysis (IOH). The data obtained convincingly demonstrate that increased IOH leads to a significant disruption of iron homeostasis, resulting in an increase in the concentration of highly toxic free radical iron in the blood plasma.
1. The pathogenetic role of free iron: The identified correlation between the degree of IOH (assessed by the level of free hemoglobin in the blood plasma at the end of CPB) and indicators of freely bound iron not complexed with carrier proteins (transferrin, hemopexin) confirms the key role of hemolysis in the release of this highly reactive and cytotoxic element. Free iron is a powerful catalyst for the Fenton and Haber-Weiss reactions, leading to the formation of reactive oxygen species (ROS) and the development of oxidative stress [16-19].
2. Potential mechanism for coronary heart disease complications after CABG: Increased plasma free iron levels are a significant predictor and/or mediator of tissue damage. Under conditions of ischemia-reperfusion, typical of cardiac surgery, excess free iron can exacerbate damage to the myocardium, vascular endothelium, and other organs, contributing to the development of complications such as myocardial dysfunction, arrhythmias, renal failure, systemic inflammatory response syndrome, and other postoperative complications frequently observed in patients with coronary heart disease after CABG. 3. Clinical implications and diagnostic potential:
- Risk stratification: Monitoring plasma free hemoglobin levels at the end of CPB, as well as changes in free-bound iron parameters, can serve as an effective tool for early stratification of patients with coronary artery disease undergoing CABG based on their risk of developing postoperative complications.
- Biomarkers: The proposed parameters can be considered as potential biomarkers for assessing the extent of damage induced by IOH and iron imbalance.
4. Prospects for therapeutic strategies: The discovered patterns open new horizons for the development of targeted intervention strategies. Possible directions include:
- CPB optimization: Implementation of CPB technologies that minimize hemolysis.
- Chelation therapy: Use of drugs that can bind excess free iron (e.g., desferoxamine) to prevent its toxic effects. • Antioxidant protection: Enhancement of antioxidant defense to neutralize ROS generated by free iron.
- Induction of carrier protein synthesis: Stimulation of transferrin and hemopexin synthesis to enhance the body's natural ability to bind free iron.
Thus, our study not only deepens our understanding of the pathogenesis of postoperative complications in patients with coronary artery disease after CABG but also points to promising avenues for personalized diagnostics and protective therapy that could significantly improve the outcomes of these complex cardiac surgeries.
Abbreviations:
CABG: coronary artery bypass grafting
IOH: intraoperative hemolysis
CAD: coronary artery disease
Conflict of Interest:
The authors declare that there are no conflicts of interest.
References
- Bokeriya, L.A. Immediate results of surgical and endovascular treatment of patients with ischemic heart disease: perioperative complications, risk factors, prognosis // L.A. Bokeriya, E.Z. Golukhova, B.G. Alekyan et al., Creative Cardiology, 2011. №1. S. 41– 60.
View at Publisher | View at Google Scholar - Maksimovich E.N. Arrhythmias as a cause of death in patients after coronary artery bypass grafting, Maksimovich E.N., Dementey A.I., Lavrinaj V.V. et al. Collection of abstracts of the XII International (XXI All-Russian) Pirogov Scientific Medical Conference of Students and Young Scientists. 2017, Moskva. S.82.
View at Publisher | View at Google Scholar - Kim, L.K. Outcomes in patients undergoing coronary artery bypass graft surgery in the United States based on hospital volume, 2007 to 2011, P. Looser, R.V. Swaminathan, R.M. Minutello et al., J. Thorac. Cardiovasc. Surg. 2016. V.151(6). P.1686 –1692.
View at Publisher | View at Google Scholar - Maksimovich, Ye. Early complications after coronary bypass operation, Maksimovich Ye., Chmara N. Abstracts of the 16th International congress of medical sciences (ICMS) for students and young doctors 11-14 May 2017. Sofia. Bulgaria. P. 254.
View at Publisher | View at Google Scholar - Kim, L. K. Outcomes in patients undergoing coronary artery bypass graft surgery in the United States based on hospital volume, 2007 to 2011// P.Looser, R.V. Swaminathan, R.M. Minutello et al., J. Thorac. Cardiovasc. Surg. 2016. V/151(6). P.1686-1692.
View at Publisher | View at Google Scholar - Korthuis, R.J. Mechanisms of I/R-induced endothelium-dependent vasodilator dysfunction/ R.J. Korthuis // Adv. Pharmacol. 2018. V.81. P.331-364.
View at Publisher | View at Google Scholar - Rother, R.P. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease, R.P. Rother [et al.]; JAMA. 2005. Vol. 293. P. 1653-1662.
View at Publisher | View at Google Scholar - Chong A. Y., Blann A. D., Lip G. Y. H. Assessment of endothelial damage and dysfunction: observations in relation to heart failure; Qjm. 2003. V. 96. №. 4. P. 253-267.
View at Publisher | View at Google Scholar - Drexler H., Hornig B. Endothelial dysfunction in human disease, Journal of molecular and cellular cardiology. 1999. V. 31. №. 1. P. 51-60. Maksimovich Ye. N. Connection of Intraoperative Hemolysis with the Development of Cardiac Rhythm Disturbances; Clinical medical research. 2025. V.14, №2. P. 28-36.
View at Publisher | View at Google Scholar - Maksimovich Ye. N. Predicting of Early Cardiovascular Complications After Coronary Artery; World Journal of medical case reports. 2025 Vol. 6, №1. P.14.
View at Publisher | View at Google Scholar - Maksimovich Nataliya Y., Maksimovich Yelizaveta N., (2025), The Relationship Between the Development of Arrhythmias and Changes in Free Hemoglobin Levels During Coronary Artery Bypass Grafting; J Clinical Research Notes, 6(2); DOI:10.31579/2690- 8816/159
View at Publisher | View at Google Scholar - Maksimovich Ye. Promotion of Intraoperative Hemolysis and Life-Threatening Complications in Surgery; ISAJMS Volume 2, Issue 2, 2025; ISA Publisher ISSN: 3049-1746. P.24-26.
View at Publisher | View at Google Scholar - Maksimovich Ye. Method for Evaluating the Risk of Cardiovascular Complications in Surgical Procedures; ISAJMS Volume 2, Issue 2, March-April, 2025; ISA Publisher ISSN: ISSN: 3049-1746. P.27-30.
View at Publisher | View at Google Scholar - Maksimovich Ye. Early Complications and Changes in Iron Levels, Markers of Oxidative Stress, and Nitric Oxide Levels in Surgery; ISA Journal of Multidisciplinary (ISAJM) Volume 2, Issue 1, Jan-Feb, 2025. P.1-5. DOI: 10.5281/zenodo.14932809
View at Publisher | View at Google Scholar - Maksimovich Ye. Mechanism of Reperfusion Syndrome and Prevention of Oxidative Stress; ISA Journal of Medical Sciences (ISAJMS) Volume 2, Issue 1, Jan-Feb 2025. P.9-11. DOI: 10.5281/zenodo.14921486/
View at Publisher | View at Google Scholar - Maksimovich Ye. Pathogenesis of Arrhythmias after Coronary Shunting; ISA Journal of Medical Sciences (ISAJMS) Volume 2, Issue 1, Jan-Feb., 2025. P.12-22. DOI: 10.5281/zenodo.14921507
View at Publisher | View at Google Scholar - Maksimovich Ye. Сhanges in the Iron Pool in Patients with Coronary Bypass Surgery; ISA Journal of Medical Sciences (ISAJMS) Volume 2, Issue 1, Jan-Feb., 2025. P.1-6. DOI: 10.5281/zenodo.14921415
View at Publisher | View at Google Scholar - Maksimovich Ye. Varying Degrees of Hemolysis during Coronary Artery Bypass Grafting Operations; ISA Journal of Medical Sciences (ISAJMS) Volume 2, Issue 1, Jan-Feb., 2025. P.1-6. DOI: 10.5281/zenodo.14921456
View at Publisher | View at Google Scholar

 Clinic
Clinic
